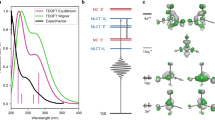
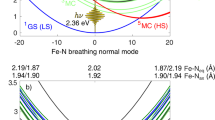
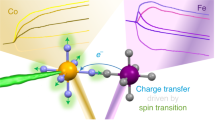
Abstract
Transition-metal complexes have long attracted interest for fundamental chemical reactivity studies and possible use in solar energy conversion1,2. Electronic excitation, ligand loss from the metal centre, or a combination of both, creates changes in charge and spin density at the metal site3,4,5,6,7,8,9,10,11 that need to be controlled to optimize complexes for photocatalytic hydrogen production8 and selective carbon–hydrogen bond activation9,10,11. An understanding at the molecular level of how transition-metal complexes catalyse reactions, and in particular of the role of the short-lived and reactive intermediate states involved, will be critical for such optimization. However, suitable methods for detailed characterization of electronic excited states have been lacking. Here we show, with the use of X-ray laser-based femtosecond-resolution spectroscopy and advanced quantum chemical theory to probe the reaction dynamics of the benchmark transition-metal complex Fe(CO)5 in solution, that the photo-induced removal of CO generates the 16-electron Fe(CO)4 species, a homogeneous catalyst12,13 with an electron deficiency at the Fe centre14,15, in a hitherto unreported excited singlet state that either converts to the triplet ground state or combines with a CO or solvent molecule to regenerate a penta-coordinated Fe species on a sub-picosecond timescale. This finding, which resolves the debate about the relative importance of different spin channels in the photochemistry of Fe(CO)5 (refs 4, 16,17,18,19 and 20), was made possible by the ability of femtosecond X-ray spectroscopy to probe frontier-orbital interactions with atom specificity. We expect the method to be broadly applicable in the chemical sciences, and to complement approaches that probe structural dynamics in ultrafast processes.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Parshall, G. W. Organometallic chemistry in homogeneous cata1ysis. Science 208, 1221–1224 (1980).
Gray, H. B. & Maverick, A. W. Solar chemistry of metal complexes. Science 214, 1201–1205 (1981).
Bengali, A. A., Bergman, R. G. & Moore, C. B. Evidence for the formation of free 16-electron species rather than solvate complexes in the ultraviolet irradiation of CpCo(CO)2 in liquefied noble gas solvents. J. Am. Chem. Soc. 117, 3879–3880 (1995).
Snee, P. T., Payne, C. K., Mebane, S. D., Kotz, K. T. & Harris, C. B. Dynamics of photosubstitution reactions of Fe(CO)5: an ultrafast infrared study of high spin reactivity. J. Am. Chem. Soc. 123, 6909–6915 (2001).
Besora, M. et al. A combined theoretical and experimental study on the role of spin states in the chemistry of Fe(CO)5 photoproducts. J. Am. Chem. Soc. 131, 3583–3592 (2009).
Juban, E. A., Smeigh, A. L., Monat, J. E. & McCusker, J. K. Ultrafast dynamics of ligand-field excited states. Coord. Chem. Rev. 250, 1783–1791 (2006).
Chergui, M. On the interplay between charge, spin and structural dynamics in transition metal complexes. Dalton Trans. 41, 13022–13029 (2012).
Heyduk, A. F. & Nocera, D. G. Hydrogen produced from hydrohalic acid solutions by a two-electron mixed-valence photocatalyst. Science 293, 1639–1641 (2001).
Arndtsen, B. A., Bergman, R. G., Mobley, T. A. & Peterson, T. H. Selective intermolecular carbon–hydrogen bond activation by synthetic metal complexes in homogeneous solution. Acc. Chem. Res. 28, 154–162 (1995).
Labinger, J. A. & Bercaw, J. E. Understanding and exploiting C–H bond activation. Nature 417, 507–514 (2002).
Bromberg, S. E. et al. The mechanism of a C–H bond activation reaction in room-temperature alkane solution. Science 278, 260–263 (1997).
Wrighton, M. S., Ginley, D. S., Schroeder, M. A. & Morse, D. L. Generation of catalysts by photolysis of transition metal complexes. Pure Appl. Chem. 41, 671–687 (1975).
Whetten, R. L., Fu, K.-J. & Grant, E. R. Pulsed-laser photocatalytic isomerization and hydrogenation of olefins. J. Am. Chem. Soc. 104, 4270–4272 (1982).
Langmuir, T. Types of valence. Science 54, 59–67 (1921).
Hoffmann, R. Building bridges between inorganic and organic chemistry. Angew. Chem. Int. Edn Engl. 21, 711–724 (1982).
Poliakoff, M. & Turner, J. J. The structure of [Fe(CO)4]—an important new chapter in a long-running story. Angew. Chem. Int. Edn Engl. 40, 2809–2812 (2001).
Trushin, S. A., Fuss, W., Kompa, K. L. & Schmid, W. E. Femtosecond dynamics of Fe(CO)5 photodissociation at 267 nm studied by transient ionization. J. Phys. Chem. A 104, 1997–2006 (2000).
Ihee, H., Cao, J. & Zewail, A. H. Ultrafast electron diffraction of transient [Fe(CO)4]: determination of molecular structure and reaction pathway. Angew. Chem. Int. Edn Engl. 40, 1532–1536 (2001).
Snee, P. T., Payne, C. K., Kotz, K. T., Yang, H. & Harris, C. B. Triplet organometallic reactivity under ambient conditions: an ultrafast UV pump/IR probe study. J. Am. Chem. Soc. 123, 2255–2264 (2001).
Ahr, B. et al. Picosecond X-ray absorption measurements of the ligand substitution dynamics of Fe(CO)5 in ethanol. Phys. Chem. Chem. Phys. 13, 5590–5599 (2011).
Nayak, S. K., Farrell, G. J. & Burkey, T. J. Photosubstitution of two iron pentacarbonyl CO's in solution via a single-photon process: dependence on dispersed ligands and role of triplet intermediates. Inorg. Chem. 33, 2236–2242 (1994).
Zhang, W. et al. Tracking excited-state charge and spin dynamics in iron coordination complexes. Nature 509, 345–348 (2014).
Bressler, C. et al. Femtosecond XANES study of the light-induced spin crossover dynamics in an iron(II) complex. Science 323, 489–492 (2009).
Huse, N. et al. Femtosecond soft X-ray spectroscopy of solvated transition-metal complexes: deciphering the interplay of electronic and structural dynamics. J. Phys. Chem. Lett. 2, 880–884 (2011).
Portius, P. et al. Unraveling the photochemistry of Fe(CO)5 in solution: observation of Fe(CO)3 and the conversion between 3Fe(CO)4 and 1Fe(CO)4(solvent). J. Am. Chem. Soc. 126, 10713–10720 (2004).
Joly, A. G. & Nelson, K. A. Metal carbonyl photochemistry in organic solvents: femtosecond transient absorption and preliminary resonance Raman spectroscopy. Chem. Phys. 152, 69–82 (1991).
Fukui, K. The role of frontier orbitals in chemical reactions. Angew. Chem. Int. Edn Engl. 21, 801–809 (1982).
Lim, M., Jackson, T. A. & Anfinrud, P. A. Binding of CO to myoglobin from a heme pocket docking site to form nearly linear Fe–C–O. Science 269, 962–966 (1995).
Nibbering, E. T. J., Fidder, H. & Pines, E. Ultrafast chemistry: using time-resolved vibrational spectroscopy for interrogation of structural dynamics. Annu. Rev. Phys. Chem. 56, 338–367 (2005).
Greaves, S. J. et al. Vibrationally quantum-state-specific reaction dynamics of H atom abstraction by CN radical in solution. Science 331, 1423–1426 (2011).
Acknowledgements
This work was supported by the Volkswagen Stiftung (M.B.) the Swedish Research Council (M.O.), the Carl Tryggers Foundation (M.O.), the Magnus Bergvall Foundation (M.O.), the Collaborative Research Centers SFB 755 and SFB 1073 (I.R., S.G., W.Q., M.S. and S.T.) and the Helmholtz Virtual Institute ‘Dynamic Pathways in Multidimensional Landscapes’. W.Z., R.W.H. and K.J.G. acknowledge support through the AMOS program within the Chemical Sciences, Geosciences, and Biosciences Division of the Office of Basic Energy Sciences, Office of Science, US Department of Energy. Portions of this research were performed on the Soft X-ray Materials Science (SXR) Instrument at the Linac Coherent Light Source (LCLS), a division of SLAC National Accelerator Laboratory and an Office of Science user facility operated by Stanford University for the US Department of Energy. The SXR Instrument is funded by a consortium whose membership includes the LCLS, Stanford University through the Stanford Institute for Materials Energy Sciences (SIMES), Lawrence Berkeley National Laboratory (LBNL), the University of Hamburg through the BMBF priority program FSP 301, and the Center for Free Electron Laser Science (CFEL).
Author information
Authors and Affiliations
Contributions
Ph.W., K.K., I.R., W.Q., M.B., S.S., D.N., W.F.S., J.J.T., F.H., S.T. and A.F. designed the experiment. Ph.W., K.K., I.R., W.Q., M.B., S.S., S.G., M.S., D.N., W.Z., R.W.H., W.F.S., J.J.T., B.K., F.H., K.J.G., S.T. and A.F. did the experiment. K.K., Ph.W., M.B. and A.F. analysed the experimental data. I.J., K.K. and M.O. performed the calculations. Ph.W., K.K. and K.J.G. wrote the manuscript with input from all authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
This file contains Supplementary Text, Supplementary Figures 1-14, Supplementary Tables 1-3, Supplementary References and Supplementary Acknowledgements. (PDF 2973 kb)
Source data
Rights and permissions
About this article
Cite this article
Wernet, P., Kunnus, K., Josefsson, I. et al. Orbital-specific mapping of the ligand exchange dynamics of Fe(CO)5 in solution. Nature 520, 78–81 (2015). https://doi.org/10.1038/nature14296
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature14296
This article is cited by
-
Probing the interplay between lattice dynamics and short-range magnetic correlations in CuGeO3 with femtosecond RIXS
npj Quantum Materials (2021)
-
Using X-ray free-electron lasers for spectroscopy of molecular catalysts and metalloenzymes
Nature Reviews Physics (2021)
-
Observation of the molecular response to light upon photoexcitation
Nature Communications (2020)
-
Probing light-driven quantum materials with ultrafast resonant inelastic X-ray scattering
Communications Physics (2020)
-
Time-resolved RIXS experiment with pulse-by-pulse parallel readout data collection using X-ray free electron laser
Scientific Reports (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.