Abstract
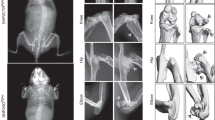
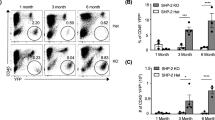
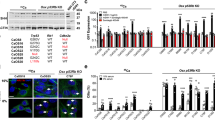
The tyrosine phosphatase SHP2, encoded by PTPN11, is required for the survival, proliferation and differentiation of various cell types1,2. Germline activating mutations in PTPN11 cause Noonan syndrome, whereas somatic PTPN11 mutations cause childhood myeloproliferative disease and contribute to some solid tumours. Recently, heterozygous inactivating mutations in PTPN11 were found in metachondromatosis, a rare inherited disorder featuring multiple exostoses, enchondromas, joint destruction and bony deformities3,4. The detailed pathogenesis of this disorder has remained unclear. Here we use a conditional knockout (floxed) Ptpn11 allele (Ptpn11fl) and Cre recombinase transgenic mice to delete Ptpn11 specifically in monocytes, macrophages and osteoclasts (lysozyme M-Cre; LysMCre) or in cathepsin K (Ctsk)-expressing cells, previously thought to be osteoclasts. LysMCre;Ptpn11fl/fl mice had mild osteopetrosis. Notably, however, CtskCre;Ptpn11fl/fl mice developed features very similar to metachondromatosis. Lineage tracing revealed a novel population of CtskCre-expressing cells in the perichondrial groove of Ranvier that display markers and functional properties consistent with mesenchymal progenitors. Chondroid neoplasms arise from these cells and show decreased extracellular signal-regulated kinase (ERK) pathway activation, increased Indian hedgehog (Ihh) and parathyroid hormone-related protein (Pthrp, also known as Pthlh) expression and excessive proliferation. Shp2-deficient chondroprogenitors had decreased fibroblast growth factor-evoked ERK activation and enhanced Ihh and Pthrp expression, whereas fibroblast growth factor receptor (FGFR) or mitogen-activated protein kinase kinase (MEK) inhibitor treatment of chondroid cells increased Ihh and Pthrp expression. Importantly, smoothened inhibitor treatment ameliorated metachondromatosis features in CtskCre;Ptpn11fl/fl mice. Thus, in contrast to its pro-oncogenic role in haematopoietic and epithelial cells, Ptpn11 is a tumour suppressor in cartilage, acting through a FGFR/MEK/ERK-dependent pathway in a novel progenitor cell population to prevent excessive Ihh production.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Change history
16 April 2014
A Correction to this paper has been published: https://doi.org/10.1038/nature13170
References
Neel, B. G., Chan, G. & Dhanji, S. in Handbook of Cell Signaling Vol. 2 (eds Bradshaw, R. A. & Dennis, E. A. ) Ch. 98, 771–809 (2009)
Grossmann, K. S., Rosario, M., Birchmeier, C. & Birchmeier, W. The tyrosine phosphatase Shp2 in development and cancer. Adv. Cancer Res. 106, 53–89 (2010)
Bowen, M. E. et al. Loss-of-function mutations in PTPN11 cause metachondromatosis, but not Ollier disease or Maffucci syndrome. PLoS Genet. 7, e1002050 (2011)
Sobreira, N. L. et al. Whole-genome sequencing of a single proband together with linkage analysis identifies a Mendelian disease gene. PLoS Genet. 6, e1000991 (2010)
Bovée, J. V., Hogendoorn, P. C., Wunder, J. S. & Alman, B. A. Cartilage tumours and bone development: molecular pathology and possible therapeutic targets. Nature Rev. Cancer 10, 481–488 (2010)
Pannier, S. & Legeai-Mallet, L. Hereditary multiple exostoses and enchondromatosis. Best Pract. Res. Clin. Rheumatol. 22, 45–54 (2008)
Pansuriya, T. C., Kroon, H. M. & Bovee, J. V. Enchondromatosis: insights on the different subtypes. Int. J. Clin. Exp. Pathol. 3, 557–569 (2010)
Tartaglia, M., Gelb, B. D. & Zenker, M. Noonan syndrome and clinically related disorders. Best Pract. Res. Clin. Endocrinol. Metab. 25, 161–179 (2011)
Saxton, T. M. et al. Abnormal mesoderm patterning in mouse embryos mutant for the SH2 tyrosine phosphatase Shp-2. EMBO J. 16, 2352–2364 (1997)
Yang, W. et al. An Shp2/SFK/Ras/Erk signaling pathway controls trophoblast stem cell survival. Dev. Cell 10, 317–327 (2006)
Clausen, B. E., Burkhardt, C., Reith, W., Renkawitz, R. & Forster, I. Conditional gene targeting in macrophages and granulocytes using LysMcre mice. Transgenic Res. 8, 265–277 (1999)
Nakamura, T. et al. Estrogen prevents bone loss via estrogen receptor α and induction of Fas ligand in osteoclasts. Cell 130, 811–823 (2007)
Dodds, R. A., Connor, J. R., Drake, F., Feild, J. & Gowen, M. Cathepsin K mRNA detection is restricted to osteoclasts during fetal mouse development. J. Bone Miner. Res. 13, 673–682 (1998)
Langenskiöld, A. Role of the ossification groove of Ranvier in normal and pathologic bone growth: a review. J. Pediatr. Orthop. 18, 173–177 (1998)
Shapiro, F., Holtrop, M. E. & Glimcher, M. J. Organization and cellular biology of the perichondrial ossification groove of ranvier: a morphological study in rabbits. J. Bone Joint Surg. Am. 59, 703–723 (1977)
Karlsson, C., Thornemo, M., Henriksson, H. B. & Lindahl, A. Identification of a stem cell niche in the zone of Ranvier within the knee joint. J. Anat. 215, 355–363 (2009)
Goldring, M. B., Tsuchimochi, K. & Ijiri, K. The control of chondrogenesis. J. Cell. Biochem. 97, 33–44 (2006)
Hopyan, S. et al. A mutant PTH/PTHrP type I receptor in enchondromatosis. Nature Genet. 30, 306–310 (2002)
Tiet, T. D. et al. Constitutive Hedgehog signaling in chondrosarcoma up-regulates tumor cell proliferation. Am. J. Pathol. 168, 321–330 (2006)
Liu, Z., Xu, J., Colvin, J. S. & Ornitz, D. M. Coordination of chondrogenesis and osteogenesis by fibroblast growth factor 18. Genes Dev. 16, 859–869 (2002)
Ohbayashi, N. et al. FGF18 is required for normal cell proliferation and differentiation during osteogenesis and chondrogenesis. Genes Dev. 16, 870–879 (2002)
Kronenberg, H. M. PTHrP and skeletal development. Ann. NY Acad. Sci. 1068, 1–13 (2006)
Murakami, S. et al. Constitutive activation of MEK1 in chondrocytes causes Stat1-independent achondroplasia-like dwarfism and rescues the Fgfr3-deficient mouse phenotype. Genes Dev. 18, 290–305 (2004)
Fenichel, I., Evron, Z. & Nevo, Z. The perichondrial ring as a reservoir for precartilaginous cells. In vivo model in young chicks’ epiphysis. Int. Orthop. 30, 353–356 (2006)
Robinson, D. et al. Fibroblast growth factor receptor-3 as a marker for precartilaginous stem cells. Clin. Orthop. Relat. Res. S163–S175 (1999)
Rodríguez, J. I., Delgado, E. & Paniagua, R. Changes in young rat radius following excision of the perichondrial ring. Calcif. Tissue Int. 37, 677–683 (1985)
Bard-Chapeau, E. A. et al. Ptpn11/Shp2 acts as a tumor suppressor in hepatocellular carcinogenesis. Cancer Cell 19, 629–639 (2011)
Hatakeyama, M. Oncogenic mechanisms of the Helicobacter pylori CagA protein. Nature Rev. Cancer 4, 688–694 (2004)
Soriano, P. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nature Genet. 21, 70–71 (1999)
Srinivas, S. et al. Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC Dev. Biol. 1, 4 (2001)
Hidaka, K. et al. Involvement of the phosphoinositide 3-kinase/protein kinase B signaling pathway in insulin/IGF-I-induced chondrogenesis of the mouse embryonal carcinoma-derived cell line ATDC5. Int. J. Biochem. Cell Biol. 33, 1094–1103 (2001)
Mohi, M. G. et al. Prognostic, therapeutic, and mechanistic implications of a mouse model of leukemia evoked by Shp2 (PTPN11) mutations. Cancer Cell 7, 179–191 (2005)
Pretzel, D. et al. Relative percentage and zonal distribution of mesenchymal progenitor cells in human osteoarthritic and normal cartilage. Arthritis Res. Ther. 13, R64 (2011)
Acknowledgements
We thank S. Kato for Ctsk-Cre mice, A. Craft for review of the manuscript, X. Wang and P. Monfils for help with histology and J. Cao for helping with μ-CT analysis. This publication was made possible by the National Institutes of Health (NIH) and the National Institute for General Medicine Sciences (NIGMS) grant no. 8P20GM103468. This work was also funded by NIH R21AR57156 (to W.Y.) and R37CA49152 (to B.G.N.), the Rhode Island Hospital Orthopaedic Foundation and a grant from the Pediatric Orthopaedic Society of North America and the Orthopaedic Research and Education Foundation (to W.Y.). B.G.N. is a Canada Research Chair, Tier 1, and is also supported in part by the Ontario Ministry of Health and Long Term Care and the Princess Margaret Cancer Foundation.
Author information
Authors and Affiliations
Contributions
W.Y. and B.G.N. conceived the project. J.W., D.M., H.L. and W.Y. carried out most of the experiments. M.D. and W.Y. conducted FACS sorting and analysis. H.L. performed the SMOi animal treatment experiment. J.W. performed gene expression and western blot analysis. M.D. and J.W. carried out bone marrow transplantation experiments with the advice of P.J.Q. M.D., H.L. and W.Y. performed CPC multi-lineage differentiation assays. Q.W. and R.T. performed histological staining and data interpretation. Q.C. and M.G.E. provided technical and intellectual support. W.Y. and B.G.N. analysed the data and wrote the manuscript with the help of all authors.
Corresponding author
Ethics declarations
Competing interests
W.Y. and B.G.N. have filed a provisional patent application on the use of Smoothened inhibitors for the treatment of metachondromatosis (Title: Hedgehog Pathway Inhibition for Cartilage Tumor and Metachondromatosis Treatment; UPA#61/614,449)
Supplementary information
Supplementary Figures
This file contains Supplementary Figures 1-9. (PDF 760 kb)
Improved Joint Function in Ctsk-KO
Video clips 1 and 2 show that Ctsk-KO mice treated with SMOi, but not Vehicle, have dramatically improved joint function. In each clip, one mouse is wild type, one is a Ctsk-KO mouse treated with SMOi, and the third is Ctsk-KO mouse treated with vehicle alone. SMOi treatment of Control mice has no apparent effect on mobility. (MOV 23455 kb)
Rights and permissions
About this article
Cite this article
Yang, W., Wang, J., Moore, D. et al. Ptpn11 deletion in a novel progenitor causes metachondromatosis by inducing hedgehog signalling. Nature 499, 491–495 (2013). https://doi.org/10.1038/nature12396
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature12396
This article is cited by
-
Molecular Heterogeneity of Osteopetrosis in India: Report of 17 Novel Variants
Indian Journal of Hematology and Blood Transfusion (2024)
-
Hedgehog signaling regulates bone homeostasis through orchestrating osteoclast differentiation and osteoclast–osteoblast coupling
Cellular and Molecular Life Sciences (2023)
-
Development and validation of machine learning models for the prediction of SH-2 containing protein tyrosine phosphatase 2 inhibitors
Molecular Diversity (2023)
-
Periosteal stem cells control growth plate stem cells during postnatal skeletal growth
Nature Communications (2022)
-
A novel lineage of osteoprogenitor cells with dual epithelial and mesenchymal properties govern maxillofacial bone homeostasis and regeneration after MSFL
Cell Research (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.