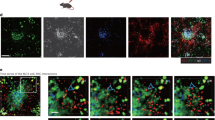
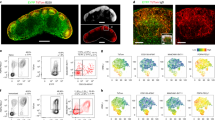
Abstract
The splenic marginal zone is a unique microenvironment where resident immune cells are exposed to the open blood circulation1,2. Even though it has an important role in responses against blood-borne antigens, lymphocyte migration in the marginal zone has not been intravitally visualized due to challenges associated with achieving adequate imaging depth in this abdominal organ. Here we develop a two-photon microscopy procedure to study marginal zone and follicular B-cell movement in the live mouse spleen. We show that marginal zone B cells are highly motile and exhibit long membrane extensions. Marginal zone B cells shuttle between the marginal zone and follicles with at least one-fifth of the cells exchanging between compartments per hour, a behaviour that explains their ability to deliver antigens rapidly from the open blood circulation to the secluded follicles. Follicular B cells also transit from follicles to the marginal zone, but unlike marginal zone B cells, they fail to undergo integrin-mediated adhesion, become caught in fluid flow and are carried into the red pulp. Follicular B-cell egress via the marginal zone is sphingosine-1-phosphate receptor-1 (S1PR1)-dependent. This study shows that marginal zone B cells migrate continually between marginal zone and follicles and establishes the marginal zone as a site of S1PR1-dependent B-cell exit from follicles. The results also show how adhesive differences of similar cells critically influence their behaviour in the same microenvironment.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Schmidt, E. E., MacDonald, I. C. & Groom, A. C. Comparative aspects of splenic microcirculatory pathways in mammals: the region bordering the white pulp. Scanning Microsc. 7, 613–628 (1993)
Mebius, R. E. & Kraal, G. Structure and function of the spleen. Nature Rev. Immunol. 5, 606–616 (2005)
Martin, F. & Kearney, J. F. Marginal zone B cells. Nature Rev. Immunol. 2, 323–335 (2002)
Pillai, S. & Cariappa, A. The follicular versus marginal zone B lymphocyte cell fate decision. Nature Rev. Immunol. 9, 767–777 (2009)
MacLennan, I. C. M., Gray, D., Kumararatne, D. S. & Bazin, H. The lymphocytes of splenic marginal zones: a distinct B-cell lineage. Immunol. Today 3, 305–307 (1982)
Lu, T. T. & Cyster, J. G. Integrin-mediated long-term B cell retention in the splenic marginal zone. Science 297, 409–412 (2002)
Guinamard, R., Okigaki, M., Schlessinger, J. & Ravetch, J. V. Absence of marginal zone B cells in Pyk-2 deficient mice define their role in the humoral response. Nature Immunol. 1, 31–36 (2000)
Ferguson, A. R., Youd, M. E. & Corley, R. B. Marginal zone B cells transport and deposit IgM-containing immune complexes onto follicular dendritic cells. Int. Immunol. 16, 1411–1422 (2004)
Cinamon, G., Zachariah, M., Lam, O. & Cyster, J. G. Follicular shuttling of marginal zone B cells facilitates antigen transport. Nature Immunol. 9, 54–62 (2008)
Arnon, T. I. et al. GRK2-dependent S1PR1 desensitization is required for lymphocytes to overcome their attraction to blood. Science 333, 1898–1903 (2011)
Kumararatne, D. S. & MacLennan, I. C. Cells of the marginal zone of the spleen are lymphocytes derived from recirculating precursors. Eur. J. Immunol. 11, 865–869 (1981)
You, Y., Zhao, H., Wang, Y. & Carter, R. H. Cutting edge: primary and secondary effects of CD19 deficiency on cells of the marginal zone. J. Immunol. 182, 7343–7347 (2009)
Hao, Z. & Rajewsky, K. Homeostasis of peripheral B cells in the absence of B cell influx from the bone marrow. J. Exp. Med. 194, 1151–1164 (2001)
Makowska, A., Faizunnessa, N. N., Anderson, P., Midtvedt, T. & Cardell, S. CD1high B cells: a population of mixed origin. Eur. J. Immunol. 29, 3285–3294 (1999)
Martin, F. & Kearney, J. F. Positive selection from newly formed to marginal zone B cells depends on the rate of clonal production, CD19, and btk. Immunity 12, 39–49 (2000)
Oliver, A. M., Martin, F., Gartland, G. L., Carter, R. H. & Kearney, J. F. Marginal zone B cells exhibit unique activation, proliferative and immunoglobulin secretory responses. Eur. J. Immunol. 27, 2366–2374 (1997)
Allen, C. D., Okada, T., Tang, H. L. & Cyster, J. G. Imaging of germinal center selection events during affinity maturation. Science 315, 528–531 (2007)
Cinamon, G. et al. Sphingosine 1-phosphate receptor 1 promotes B cell localization in the splenic marginal zone. Nature Immunol. 5, 713–720 (2004)
Woolf, E. et al. Lymph node chemokines promote sustained T lymphocyte motility without triggering stable integrin adhesiveness in the absence of shear forces. Nature Immunol. 8, 1076–1085 (2007)
Boscacci, R. T. et al. Comprehensive analysis of lymph node stroma-expressed Ig superfamily members reveals redundant and nonredundant roles for ICAM-1, ICAM-2, and VCAM-1 in lymphocyte homing. Blood 116, 915–925 (2010)
Schwab, S. R. & Cyster, J. G. Finding a way out: lymphocyte egress from lymphoid organs. Nature Immunol. 8, 1295–1301 (2007)
Rosen, H., Sanna, M. G., Cahalan, S. M. & Gonzalez-Cabrera, P. J. Tipping the gatekeeper: S1P regulation of endothelial barrier function. Trends Immunol. 28, 102–107 (2007)
Morris, M. A. et al. Transient T cell accumulation in lymph nodes and sustained lymphopenia in mice treated with FTY720. Eur. J. Immunol. 35, 3570–3580 (2005)
Schumann, K. et al. Immobilized chemokine fields and soluble chemokine gradients cooperatively shape migration patterns of dendritic cells. Immunity 32, 703–713 (2010)
Barral, P., Sanchez-Nino, M. D., van Rooijen, N., Cerundolo, V. & Batista, F. D. The location of splenic NKT cells favours their rapid activation by blood-borne antigen. EMBO J. 31, 2378–2390 (2012)
Suzuki, K., Grigorova, I., Phan, T. G., Kelly, L. & Cyster, J. G. Visualizing B cell capture of cognate antigen from follicular dendritic cells. J. Exp. Med. 206, 1485–1493 (2009)
McDole, J. R. et al. Goblet cells deliver luminal antigen to CD103+ dendritic cells in the small intestine. Nature 483, 345–349 (2012)
Rickert, R. C., Rajewsky, K. & Roes, J. Impairment of T-cell-dependent B-cell responses and B-1 cell development in CD19-deficient mice. Nature 376, 352–355 (1995)
Molina, H. et al. Markedly impaired humoral immune response in mice deficient in complement receptors 1 and 2. Proc. Natl Acad. Sci. USA 93, 3357–3361 (1996)
Cinamon, G., Zachariah, M. A., Lam, O. M., Foss, F. W., Jr & Cyster, J. G. Follicular shuttling of marginal zone B cells facilitates antigen transport. Nature Immunol. 9, 54–62 (2008)
Pereira, J. P., An, J., Xu, Y., Huang, Y. & Cyster, J. G. Cannabinoid receptor 2 mediates the retention of immature B cells in bone marrow sinusoids. Nature Immunol. 10, 403–411 (2009)
Hargreaves, D. C. et al. A coordinated change in chemokine responsiveness guides plasma cell movements. J. Exp. Med. 194, 45–56 (2001)
Phan, T. G., Grigorova, I., Okada, T. & Cyster, J. G. Subcapsular encounter and complement-dependent transport of immune complexes by lymph node B cells. Nature Immunol. 8, 992–1000 (2007)
Kraal, G., Schornagel, K., Streeter, P. R., Holzmann, B. & Butcher, E. C. Expression of the mucosal vascular addressin, MAdCAM-1, on sinus-lining cells in the spleen. Am. J. Pathol. 147, 763–771 (1995)
Kraal, G. & Mebius, R. New insights into the cell biology of the marginal zone of the spleen. Int. Rev. Cytol. 250, 175–215 (2006)
Green, J. A. & Cyster, J. G. S1PR2 links germinal center confinement and growth regulation. Immunol. Rev. 247, 36–51 (2012)
Arnon, T. I. et al. GRK2-dependent S1PR1 desensitization is required for lymphocytes to overcome their attraction to blood. Science 333, 1898–1903 (2011)
Lo, C. G., Xu, Y., Proia, R. L. & Cyster, J. G. Cyclical modulation of sphingosine-1-phosphate receptor 1 surface expression during lymphocyte recirculation and relationship to lymphoid organ transit. J. Exp. Med. 201, 291–301 (2005)
Acknowledgements
We thank M. Miller for help with the microscope stage mount, T. Phan for advice regarding mouse surgery, J. An for technical assistance and O. M Bannard, J. R. Muppidi, M. Barnes and A. Reboldi for comments on the manuscript. T.I.A. was supported by a Jane Coffin Child’s fellowship and J.G.C. is an Investigator of the Howard Hughes Medical Institute. This work was supported in part by National Institutes of Health grant AI74847.
Author information
Authors and Affiliations
Contributions
T.I.A. and J.G.C. conceived and designed the experiments. T.I.A. performed the experiments. R.M.H. helped with some of the quantitative image analysis. I.L.G. helped with early aspects of the spleen surgery procedure. T.I.A. and J.G.C. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Figures
This file contains Supplementary Figures 1-7. (PDF 7380 kb)
Intravital TPLSM of MZ B cell migration in the spleen (~25 min)
Time lapse image sequence (57μm thick z stack) showing MZ B cells (GFP+, green) migrating in the spleen. MZ is identified by labeling MZ macrophages (MZM) with PE-IC (red). Example 1, MZ B cells are large and motile in both MZ and FO compartments and some show a dendritic morphology. Example 2, movie zooms on MZ B cells migrating between MZM and in the FO. Many MZ B cells are in close contact with MZM. The volume image is displayed using normal shading to show the 3D shape of the cells. Elapsed time is shown as hrs:min:sec. Data represents more than seven experiments. (MPG 5174 kb)
Intravital imaging of MZ B cell migration in the spleen 2 (~25 min)
Time lapse image sequence (39μm thick z stack) of spleen showing three typical behaviors of MZ B cells (GFP+, green) with respect to the MZ (MZM coated with PE-IC, red). White circle and arrow highlight a cell moving from the FO towards the MZ. Blue circle shows a cell that moves from the MZ towards the FO. Yellow dashed circle shows an example of a cell that remains confined to the MZ-FO interface by a membrane process (yellow arrow) while the cell body stretches back and forth between zones. The three cells are further highlighted by a white shadow. Many of the cells show long tails (~40μm). Image is displayed using normal shading. Elapsed time is shown as hrs:min:sec. Data are representative of more than seven experiments. (MPG 2726 kb)
Intravital imaging of MZ B cells migration before and after FTY720 treatment (~21min)
Time lapse image sequence (30μm thick z stack) of spleen showing MZ B cells (GFP+, green) migrating within the same follicle before (left image) or after (right image) injection with FTY720. Movie starts with a still image showing MZ B cells distribution in relation to MZM (PE-ICs, red). White dotted line indicates approximate MZ-FO interface. The image is displayed using normal shading to show the 3D shape of the cells. Elapsed time is shown as hrs:min:sec. Data represents one out of two experiments. (MPG 1634 kb)
MZ B cells move from the MZ into the FO
Movie shows two example (24min) time lapse image sequences (57μm thick z stacks) from intravital TPLSM of spleen showing MZ B cells (GFP, green) moving from the MZ to the FO. Grey surface was generated in Imaris using PE-IC labeled MZM and the red dashed line represents the MZ-FO interface. In example 2, cell is shown to crawl for a short time across the MZ-FO boundary before making a sharp turn and entering the FO. The cell body appears to stretch at the time of crossing. Pink lines indicate entire tracked paths of the cells. White arrows point at the crossing cell. Elapsed time is shown as hrs:min:sec. Data are representative of more than seven experiments. (MPG 3217 kb)
MZ B cell moves from the FO to the MZ
Two example time lapse image sequences (69μm thick z stacks) from intravital TPLSM of spleen showing examples of MZ B cells (GFP, green) moving from the FO to the MZ. Grey surface was generated in Imaris using PE-IC labeled MZM and the red dashed line represents the MZ-FO interface. In example 1 the MZ B cell pauses at the FO boundary for ~10 min before crossing to the other side. In example 2, the cell pauses for ~5 min at the FO boundary before crossing to the MZ. In the MZ, the cells continue to migrate in parallel to the FO-MZ interface. Pink lines indicate entire tracked paths of the cells. White arrows point at the crossing cell. Elapsed time is shown as hrs:min:sec. Data are representative of more than seven experiments. (MPG 1837 kb)
FO B cells migration in the red pulp (RP) (27min)
Time lapse image sequence (78μm) from intravital TPLSM of spleen showing FO B cells (green) migrating in a FO surrounded by PE-IC coated MZM (red) and an adjacent RP. In contrast to the FO, in the RP most cells alternate between stationary and fast movement. White dashed circles show examples of cells that either pause or move fast in a directional path away from the FO. White circled cells are further highlighted with light blue surfaces. Green dashed circles show examples of cells that remain stationary for the duration of the movie. Blue circle shows a relatively rare example of a cell that shows migratory behavior similar to cells in the FO. Elapsed time is shown as hrs:min:sec. Data are representative of more than eight experiments. (MPG 1297 kb)
FO B cells exit via the MZ
Movie shows three example time lapse image sequences of intravital TPLSM of spleen showing FO B cells (green) moving from FO to MZ. The movie begins with a time lapse image sequence (51μm thick z stack) showing both the FO B cells (GFP, green) and the PE-IC labeled MZM (red). The white dashed circle shows a FO B cell that migrates in the FO for few minutes before jumping across the FO-MZ boundary. After crossing the cell becomes immobile for the last 8 min of the movie. White line indicates entire tracked path of the cell. In the next two image sequences (54μm and 60μm thick z stacks, respectively) FO B cells (CFSE labeled, green) are shown moving from FO to MZ, with the FO-MZ boundary shown by a grey surface. The red dashed line represents the MZ-FO interface. In example 1, the blue dashed circle shows a cell that jumps across the FO-MZ boundary. Example 2 shows two FO B cells (yellow and blue dashed circles) that reach the boundary of the FO at approximately the same time and appear to jump across the MZ-FO interface simultaneously. After crossing, the cells continue to either move fast or pause similarly to the movement observed in the RP. Red line indicate entire tracked path of the cell. Circled cells are further highlighted by a light blue surface. Elapsed time is shown as hrs:min:sec. Data are representative of more than eight experiments. (MPG 4075 kb)
MZ B cell movement after integrin blockade
Three time lapse image sequences (51μm thick z stacks) from intravital TPLSM of spleen 2 hr after treatment with integrin blocking antibodies (as in Figure 4i-l) showing examples of MZ B cell (GFP, green) movement in the spleen. The first two examples show MZ B cells moving from FO to the MZ after integrin blockade. Red dashed line represents the MZ-FO interface. In both examples the MZ B cells show a meandering migration within the FO but upon crossing into the MZ they show fast tangential movements, consistent with having been caught by flow. Pink lines indicate entire tracked paths of the cells. White arrows point at the crossing cell. Third time lapse image sequence shows GFP+ MZ B cells movement in the MZ (PE-ICs, red) 2 hrs after integrin blockade. White arrow highlights a MZ B cell that was initially adherent but gradually becomes displaced and rapidly disappears from view. Blue arrow highlights another MZ B cell that exhibits mixed adhesive and jumping movements and travels into the red pulp. The volume image is displayed using normal shading to show the 3D shape of the cells. Elapsed time is shown as hrs:min:sec. Data are representative of more than five experiments. (MPG 6701 kb)
S1PR1 is required for FO B cells to egress from FO (49min)
Time lapse image sequence (90μm thick z stack) from intravital TPLSM of spleen showing WT FO B cells (CMTMR labeled, red) and S1PR1 KO FO B cells (CFSE, green) migrating in the FO. Follicles are identified by the high density of WT and KO B cell tracks (white lines). Blue lines show tracks of WT B cells that were observed leaving the FO. Green line shows a single S1PR KO B cell that was detected moving away from the FO. Elapsed time is shown as hrs:min:sec. Data are representative of more than eight experiments. (MPG 4833 kb)
Rights and permissions
About this article
Cite this article
Arnon, T., Horton, R., Grigorova, I. et al. Visualization of splenic marginal zone B-cell shuttling and follicular B-cell egress. Nature 493, 684–688 (2013). https://doi.org/10.1038/nature11738
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature11738
This article is cited by
-
Notch2 controls developmental fate choices between germinal center and marginal zone B cells upon immunization
Nature Communications (2024)
-
Dynamic encounters with red blood cells trigger splenic marginal zone B cell retention and function
Nature Immunology (2024)
-
Splenic stromal niches in homeostasis and immunity
Nature Reviews Immunology (2023)
-
Conserved stromal–immune cell circuits secure B cell homeostasis and function
Nature Immunology (2023)
-
Common and Uncommon CT Findings in CVID-Related GL-ILD: Correlations with Clinical Parameters, Therapeutic Decisions and Potential Implications in the Differential Diagnosis
Journal of Clinical Immunology (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.