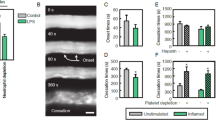
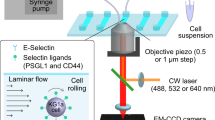
Abstract
Most leukocytes can roll along the walls of venules at low shear stress (1 dyn cm−2), but neutrophils have the ability to roll at tenfold higher shear stress in microvessels in vivo1,2. The mechanisms involved in this shear-resistant rolling are known to involve cell flattening3 and pulling of long membrane tethers at the rear4,5,6. Here we show that these long tethers do not retract as postulated6,7, but instead persist and appear as ‘slings’ at the front of rolling cells. We demonstrate slings in a model of acute inflammation in vivo and on P-selectin in vitro, where P-selectin-glycoprotein-ligand-1 (PSGL-1) is found in discrete sticky patches whereas LFA-1 is expressed over the entire length on slings. As neutrophils roll forward, slings wrap around the rolling cells and undergo a step-wise peeling from the P-selectin substrate enabled by the failure of PSGL-1 patches under hydrodynamic forces. The ‘step-wise peeling of slings’ is distinct from the ‘pulling of tethers’ reported previously4,5,6,8. Each sling effectively lays out a cell-autonomous adhesive substrate in front of neutrophils rolling at high shear stress during inflammation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Firrell, J. C. & Lipowsky, H. H. Leukocyte margination and deformation in mesenteric venules of rat. Am. J. Physiol. Heart Circ. Physiol. 256, H1667–H1674 (1989)
Sundd, P., Pospieszalska, M. K., Cheung, L. S., Konstantopoulos, K. & Ley, K. Biomechanics of leukocyte rolling. Biorheology 48, 1–35 (2011)
Damiano, E. R., Westheider, J., Tozeren, A. & Ley, K. Variation in the velocity, deformation, and adhesion energy density of leukocytes rolling within venules. Circ. Res. 79, 1122–1130 (1996)
Sundd, P. et al. Quantitative dynamic footprinting microscopy reveals mechanisms of neutrophil rolling. Nature Methods 7, 821–824 (2010)
Ramachandran, V., Williams, M., Yago, T., Schmidtke, D. W. & McEver, R. P. Dynamic alterations of membrane tethers stabilize leukocyte rolling on P-selectin. Proc. Natl Acad. Sci. USA 101, 13519–13524 (2004)
Schmidtke, D. W. & Diamond, S. L. Direct observation of membrane tethers formed during neutrophil attachment to platelets or P-selectin under physiological flow. J. Cell Biol. 149, 719–730 (2000)
Shao, J.-Y. in Leukocyte Adhesion (ed. Ley, K. ) Ch. 2, 25–45 (Academic, 2009).
Pospieszalska, M. K., Lasiecka, I. & Ley, K. Cell protrusions and tethers: a unified approach. Biophys. J. 100, 1697–1707 (2011)
Hocdé, S. A., Hyrien, O. & Waugh, R. E. Cell adhesion molecule distribution relative to neutrophil surface topography assessed by TIRFM. Biophys. J. 97, 379–387 (2009)
Moore, K. L. et al. P-selectin glycoprotein ligand-1 mediates rolling of human neutrophils on P-selectin. J. Cell Biol. 128, 661–671 (1995)
Waugh, R. E. in Leukocyte Adhesion (ed. Ley, K. ) Ch. 1, 3–24 (Academic, 2009).
Sundd, P. et al. Live cell imaging of paxillin in rolling neutrophils by dual-color quantitative dynamic footprinting. Microcirculation 18, 361–372 (2011)
Faust, N., Varas, F., Kelly, L. M., Heck, S. & Graf, T. Insertion of enhanced green fluorescent protein into the lysozyme gene creates mice with green fluorescent granulocytes and macrophages. Blood 96, 719–726 (2000)
Pendl, G. G. et al. Immature mouse dendritic cells enter inflamed tissue, a process that requires E- and P-selectin, but not P-selectin glycoprotein ligand 1. Blood 99, 946–956 (2002)
Springer, T. A. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell 76, 301–314 (1994)
Xu, H. et al. Characterization of murine intercellular adhesion molecule-2. J. Immunol. 156, 4909–4914 (1996)
Zarbock, A., Lowell, C. A. & Ley, K. Spleen tyrosine kinase Syk is necessary for E-selectin-induced αLβ2 integrin-mediated rolling on intercellular adhesion molecule-1. Immunity 26, 773–783 (2007)
Lawrence, M. B. & Springer, T. A. Leukocytes roll on a selectin at physiological flow rates: distinction from and prerequisite for adhesion through integrins. Cell 65, 859–873 (1991)
Kuwano, Y., Spelten, O., Zhang, H., Ley, K. & Zarbock, A. Rolling on E- or P-selectin induces the extended but not high-affinity conformation of LFA-1 in neutrophils. Blood 116, 617–624 (2010)
Woodfin, A. et al. The junctional adhesion molecule JAM-C regulates polarized transendothelial migration of neutrophils in vivo. Nature Immunol. 12, 761–769 (2011)
Huang, M. T. et al. ICAM-2 mediates neutrophil transmigration in vivo: evidence for stimulus specificity and a role in PECAM-1-independent transmigration. Blood 107, 4721–4727 (2006)
Marshall, B. T. et al. Direct observation of catch bonds involving cell-adhesion molecules. Nature 423, 190–193 (2003)
Xia, L. et al. P-selectin glycoprotein ligand-1-deficient mice have impaired leukocyte tethering to E-selectin under flow. J. Clin. Invest. 109, 939–950 (2002)
Ding, Z. M. et al. Relative contribution of LFA-1 and Mac-1 to neutrophil adhesion and migration. J. Immunol. 163, 5029–5038 (1999)
Hattori, R., Hamilton, K., Fugate, R., McEver, R. & Sims, P. Stimulated secretion of endothelial von Willebrand factor is accompanied by rapid redistribution to the cell surface of the intracellular granule membrane protein GMP-140. J. Biol. Chem. 264, 7768–7771 (1989)
Doyle, E. L. et al. CD63 is an essential cofactor to leukocyte recruitment by endothelial P-selectin. Blood 118, 4265–4273 (2011)
Atarashi, K., Hirata, T., Matsumoto, M., Kanemitsu, N. & Miyasaka, M. Rolling of Th1 cells via P-selectin glycoprotein ligand-1 stimulates LFA-1-mediated cell binding to ICAM-1. J. Immunol. 174, 1424–1432 (2005)
Tojo, A. et al. Nitric oxide generated by nNOS in the macula densa regulates the afferent arteriolar diameter in rat kidney. Med. Electron Microsc. 37, 236–241 (2004)
Ley, K. et al. Sequential contribution of L- and P-selectin to leukocyte rolling in vivo. J. Exp. Med. 181, 669–675 (1995)
Kunkel, E. J. et al. Absence of trauma-induced leukocyte rolling in mice deficient in both P-selectin and intercellular adhesion molecule 1. J. Exp. Med. 183, 57–65 (1996)
Sperandio, M. et al. Severe impairment of leukocyte rolling in venules of core 2 glucosaminyltransferase-deficient mice. Blood 97, 3812–3819 (2001)
Schmid-Schönbein, G. W. Leukocyte biophysics. An invited review. Cell Biophys. 17, 107–135 (1990)
Pospieszalska, M. K. & Ley, K. in Leukocyte Adhesion (ed. Ley, K. ) Ch. 8 221–296 (Academic, 2009)
Brochard-Wyart, F., Borghi, N., Cuvelier, D. & Nassoy, P. Hydrodynamic narrowing of tubes extruded from cells. Proc. Natl Acad. Sci. USA 103, 7660–7663 (2006)
Goldman, A. J., Cox, R. G. & Brenner, H. Slow viscous motion of a sphere parallel to a plane wall. II. Couette flow. Chem. Eng. Sci. 22, 653–660 (1967)
Pospieszalska, M. K., Zarbock, A., Pickard, J. E. & Ley, K. Event-tracking model of adhesion identifies load-bearing bonds in rolling leukocytes. Microcirculation 16, 115–130 (2009)
Krasik, E. F. & Hammer, D. A. A semianalytic model of leukocyte rolling. Biophys. J. 87, 2919–2930 (2004)
Goldman, A. J., Cox, R. G. & Brenner, H. Slow viscous motion of a sphere parallel to a plane wall. I. Motion through a quiescent fluid. Chem. Eng. Sci. 22, 637–651 (1967)
Wilcox, R. R. New Statistical Procedures For The Social Sciences: Modern Solutions To Basic Problems (Lawrence Erlbaum Associates, 1987)
Acknowledgements
The authors thank A. Zychlinsky for comments and reading the manuscript. This study was supported by the NCRP-Scientist Development Grant 11SDG7340005 from the American Heart Association (P.S.), WSA postdoctoral fellowship 10POST4160142-01 from American Heart Association (E.K.K.) and NIH EB 02185 (K.L.).
Author information
Authors and Affiliations
Contributions
P.S. performed all the experiments and image analysis. E.G. and A.G. designed the microfluidic device. M.K.P. calculated the fraction of bond force and torque shared by slings and tethers. E.K.K. was involved in culturing of Th1 CD4 T cells. Y.K. and S.F performed the scanning electron microscopy. P.S. and K.L. wrote the manuscript. K.L. supervised the project. All authors discussed the results and commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
This file contains Supplementary Figures 1-28, Supplementary Notes 1-4 and legends for Supplementary Movies 1-9. (PDF 9702 kb)
Supplementary Movie 1
Slings formed by DiI-stained mouse bone marrow neutrophil rolling on P-selectin - see Supplementary Information file for full legend. (MOV 1664 kb)
Supplementary Movie 2
Sling formed by an EGFP neutrophil rolling on P-selectin in whole blood of Lyz2-EGFP mouse - see Supplementary Information file for full legend. (MOV 918 kb)
Supplementary Movie 3
Wrapping of slings around a DiI-stained mouse bone marrow neutrophil rolling on P-selectin - see Supplementary Information file for full legend. (MOV 2828 kb)
Supplementary Movie 4
Wrapping of sling by a leukocyte rolling in the cremaster venule of a WT mouse - see Supplementary Information file for full legend. (MOV 216 kb)
Supplementary Movie 5
Sling formation by a leukocyte rolling in the cremaster venule of a WT mouse. Image processed to reveal sling - see Supplementary Information file for full legend. (MOV 75 kb)
Supplementary Movie 6
Tether (arrowhead) swings over to become a sling (arrow) - see Supplementary Information file for full legend. (MOV 222 kb)
Supplementary Movie 7
Tether (arrowhead) swings over to become a sling (arrow) - see Supplementary Information file for full legend. (MOV 1855 kb)
Supplementary Movie 8
Step-wise peeling of a sling. PSGL-1 patches (red spots) visible on sling (green) - see Supplementary Information file for full legend. (MOV 3116 kb)
Supplementary Movie 9
Step-wise peeling of a sling. PSGL-1 patches (red spots) visible on sling (green) - see Supplementary Information file for full legend. (MOV 2980 kb)
Rights and permissions
About this article
Cite this article
Sundd, P., Gutierrez, E., Koltsova, E. et al. ‘Slings’ enable neutrophil rolling at high shear. Nature 488, 399–403 (2012). https://doi.org/10.1038/nature11248
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature11248
This article is cited by
-
Probing mechanical interaction of immune receptors and cytoskeleton by membrane nanotube extraction
Scientific Reports (2023)
-
An Intriguing Structural Modification in Neutrophil Migration Across Blood Vessels to Inflammatory Sites: Progress in the Core Mechanisms
Cell Biochemistry and Biophysics (2023)
-
Multiphoton intravital microscopy of rodents
Nature Reviews Methods Primers (2022)
-
Hemodynamic analysis for stenosis microfluidic model of thrombosis with refined computational fluid dynamics simulation
Scientific Reports (2021)
-
Single-molecule imaging and microfluidic platform reveal molecular mechanisms of leukemic cell rolling
Communications Biology (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.