Abstract
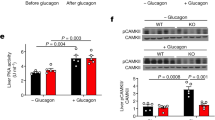
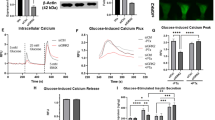
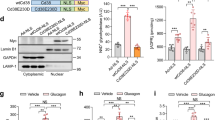
In the fasted state, increases in circulating glucagon promote hepatic glucose production through induction of the gluconeogenic program. Triggering of the cyclic AMP pathway increases gluconeogenic gene expression via the de-phosphorylation of the CREB co-activator CRTC2 (ref. 1). Glucagon promotes CRTC2 dephosphorylation in part through the protein kinase A (PKA)-mediated inhibition of the CRTC2 kinase SIK2. A number of Ser/Thr phosphatases seem to be capable of dephosphorylating CRTC2 (refs 2, 3), but the mechanisms by which hormonal cues regulate these enzymes remain unclear. Here we show in mice that glucagon stimulates CRTC2 dephosphorylation in hepatocytes by mobilizing intracellular calcium stores and activating the calcium/calmodulin-dependent Ser/Thr-phosphatase calcineurin (also known as PP3CA). Glucagon increased cytosolic calcium concentration through the PKA-mediated phosphorylation of inositol-1,4,5-trisphosphate receptors (InsP3Rs), which associate with CRTC2. After their activation, InsP3Rs enhanced gluconeogenic gene expression by promoting the calcineurin-mediated dephosphorylation of CRTC2. During feeding, increases in insulin signalling reduced CRTC2 activity via the AKT-mediated inactivation of InsP3Rs. InsP3R activity was increased in diabetes, leading to upregulation of the gluconeogenic program. As hepatic downregulation of InsP3Rs and calcineurin improved circulating glucose levels in insulin resistance, these results demonstrate how interactions between cAMP and calcium pathways at the level of the InsP3R modulate hepatic glucose production under fasting conditions and in diabetes.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Altarejos, J. Y. & Montminy, M. CREB and the CRTC co-activators: sensors for hormonal and metabolic signals. Nature Rev. Mol. Cell Biol. 12, 141–151 (2011)
Yoon, Y. S. et al. Suppressor of MEK null (SMEK)/protein phosphatase 4 catalytic subunit (PP4C) is a key regulator of hepatic gluconeogenesis. Proc. Natl Acad. Sci. USA 107, 17704–17709 (2010)
Screaton, R. A. et al. The CREB coactivator TORC2 functions as a calcium- and cAMP-sensitive coincidence detector. Cell 119, 61–74 (2004)
Hogan, P. G., Chen, L., Nardone, J. & Rao, A. Transcriptional regulation by calcium, calcineurin, and NFAT. Genes Dev. 17, 2205–2232 (2003)
Ferris, C. D., Huganir, R. L., Bredt, D. S., Cameron, A. M. & Snyder, S. H. Inositol trisphosphate receptor: phosphorylation by protein kinase C and calcium calmodulin-dependent protein kinases in reconstituted lipid vesicles. Proc. Natl Acad. Sci. USA 88, 2232–2235 (1991)
Volpe, P. & Alderson-Lang, B. H. Regulation of inositol 1,4,5-trisphosphate-induced Ca2+ release. II. Effect of cAMP-dependent protein kinase. Am. J. Physiol. 258, C1086–C1091 (1990)
Bird, G. S., Burgess, G. M. & Putney, J. W., Jr Sulfhydryl reagents and cAMP-dependent kinase increase the sensitivity of the inositol 1,4,5-trisphosphate receptor in hepatocytes. J. Biol. Chem. 268, 17917–17923 (1993)
Patterson, R. L., Boehning, D. & Snyder, S. H. Inositol 1,4,5-trisphosphate receptors as signal integrators. Annu. Rev. Biochem. 73, 437–465 (2004)
Futatsugi, A. et al. IP3 receptor types 2 and 3 mediate exocrine secretion underlying energy metabolism. Science 309, 2232–2234 (2005)
Cruz, L. N. et al. Regulation of multidrug resistance-associated protein 2 by calcium signaling in mouse liver. Hepatology 52, 327–337 (2010)
Szado, T. et al. Phosphorylation of inositol 1,4,5-trisphosphate receptors by protein kinase B/AKT inhibits Ca2+ release and apoptosis. Proc. Natl Acad. Sci. USA 105, 2427–2432 (2008)
Tovey, S. C. et al. Regulation of inositol 1,4,5-trisphosphate receptors by cAMP independent of cAMP-dependent protein kinase. J. Biol. Chem. 285, 12979–12989 (2010)
Wakelam, M. J., Murphy, G. J., Hruby, V. J. & Houslay, M. D. Activation of two signal-transduction systems in hepatocytes by glucagon. Nature 323, 68–71 (1986)
Yoon, J. et al. Control of hepatic gluconeogenesis through the transcriptional coactivator PGC-1. Nature 413, 131–138 (2001)
Herzig, S. et al. CREB regulates hepatic gluconeogenesis via the co-activator PGC-1. Nature 413, 179–183 (2001)
Wu, Z. et al. Transducer of regulated CREB-binding proteins (TORCs) induce PGC-1α transcription and mitochondrial biogenesis in muscle cells. Proc. Natl Acad. Sci. USA 103, 14379–14384 (2006)
Dentin, R. et al. Insulin modulates gluconeogenesis by inhibition of the coactivator TORC2. Nature 449, 366–369 (2007)
Wang, Y., Vera, L., Fischer, W. H. & Montminy, M. The CREB coactivator CRTC2 links hepatic ER stress and fasting gluconeogenesis. Nature 460, 534–537 (2009)
Jansson, D. et al. Glucose controls CREB activity in islet cells via regulated phosphorylation of TORC2. Proc. Natl Acad. Sci. USA 105, 10161–10166 (2008)
Liu, Y. et al. A fasting inducible switch modulates gluconeogenesis via activator/coactivator exchange. Nature 456, 269–273 (2008)
Wang, B. et al. A hormone-dependent module regulating energy balance. Cell 145, 596–606 (2011)
Acknowledgements
This work was supported by National Institutes of Health grants R01-DK049777, R01-DK083834 and R01-DK091618 (M.M.), HL087123 (I.T.), the Kieckhefer Foundation, The Clayton Foundation for Medical Research, and the Leona M. and Harry B. Helmsley Charitable Trust.
Author information
Authors and Affiliations
Contributions
Y.W., I.T. and M.M. designed and interpreted the experiments. Y.W., G.L., J.C.P., R.S. and J.G. carried out the experimental work. Y.W., K.O. and J.C. characterized glucose metabolism in Insp3r2 knockout mice. W.H.F. performed proteomic studies, and Y.W. and M.M. wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Figures
This file contains Supplementary Figures 1-12. (PDF 2671 kb)
Rights and permissions
About this article
Cite this article
Wang, Y., Li, G., Goode, J. et al. Inositol-1,4,5-trisphosphate receptor regulates hepatic gluconeogenesis in fasting and diabetes. Nature 485, 128–132 (2012). https://doi.org/10.1038/nature10988
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature10988
This article is cited by
-
Famsin, a novel gut-secreted hormone, contributes to metabolic adaptations to fasting via binding to its receptor OLFR796
Cell Research (2023)
-
CD38/ADP-ribose/TRPM2-mediated nuclear Ca2+ signaling is essential for hepatic gluconeogenesis in fasting and diabetes
Experimental & Molecular Medicine (2023)
-
Phosphoinositides and intracellular calcium signaling: novel insights into phosphoinositides and calcium coupling as negative regulators of cellular signaling
Experimental & Molecular Medicine (2023)
-
The functions of Ca2+/calmodulin-dependent protein kinase II (CaMKII) in diabetes progression
Journal of Cell Communication and Signaling (2023)
-
Hepatic p38α MAPK controls gluconeogenesis via FOXO1 phosphorylation at S273 during glucagon signalling in mice
Diabetologia (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.