Abstract
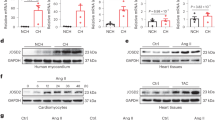
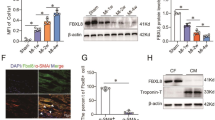
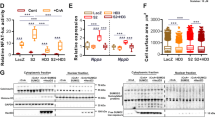
The calcium-transporting ATPase ATP2A2, also known as SERCA2a, is a critical ATPase responsible for Ca2+ re-uptake during excitation–contraction coupling. Impaired Ca2+ uptake resulting from decreased expression and reduced activity of SERCA2a is a hallmark of heart failure1. Accordingly, restoration of SERCA2a expression by gene transfer has proved to be effective in improving cardiac function in heart-failure patients2, as well as in animal models3. The small ubiquitin-related modifier (SUMO) can be conjugated to lysine residues of target proteins4, and is involved in many cellular processes5. Here we show that SERCA2a is SUMOylated at lysines 480 and 585 and that this SUMOylation is essential for preserving SERCA2a ATPase activity and stability in mouse and human cells. The levels of SUMO1 and the SUMOylation of SERCA2a itself were greatly reduced in failing hearts. SUMO1 restitution by adeno-associated-virus-mediated gene delivery maintained the protein abundance of SERCA2a and markedly improved cardiac function in mice with heart failure. This effect was comparable to SERCA2A gene delivery. Moreover, SUMO1 overexpression in isolated cardiomyocytes augmented contractility and accelerated Ca2+ decay. Transgene-mediated SUMO1 overexpression rescued cardiac dysfunction induced by pressure overload concomitantly with increased SERCA2a function. By contrast, downregulation of SUMO1 using small hairpin RNA (shRNA) accelerated pressure-overload-induced deterioration of cardiac function and was accompanied by decreased SERCA2a function. However, knockdown of SERCA2a resulted in severe contractile dysfunction both in vitro and in vivo, which was not rescued by overexpression of SUMO1. Taken together, our data show that SUMOylation is a critical post-translational modification that regulates SERCA2a function, and provide a platform for the design of novel therapeutic strategies for heart failure.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Meyer, M. et al. Alterations of sarcoplasmic reticulum proteins in failing human dilated cardiomyopathy. Circulation 92, 778–784 (1995)
Jessup, M. et al. Calcium upregulation by percutaneous administration of gene therapy in cardiac disease (CUPID): a phase 2 trial of intracoronary gene therapy of sarcoplasmic reticulum Ca2+-ATPase in patients with advanced heart failure. Circulation 124, 304–313 (2011)
Kawase, Y. et al. Reversal of cardiac dysfunction after long-term expression of SERCA2a by gene transfer in a pre-clinical model of heart failure. J. Am. Coll. Cardiol. 51, 1112–1119 (2008)
Johnson, E. S. Protein modification by SUMO. Annu. Rev. Biochem. 73, 355–382 (2004)
Geiss-Friedlander, R. & Melchior, F. Concepts in sumoylation: a decade on. Nature Rev. Mol. Cell Biol. 8, 947–956 (2007)
Adachi, T. et al. S-Glutathiolation by peroxynitrite activates SERCA during arterial relaxation by nitric oxide. Nature Med. 10, 1200–1207 (2004)
Knyushko, T. V., Sharov, V. S., Williams, T. D., Schoneich, C. & Bigelow, D. J. 3-Nitrotyrosine modification of SERCA2a in the aging heart: a distinct signature of the cellular redox environment. Biochemistry 44, 13071–13081 (2005)
Sampson, D. A., Wang, M. & Matunis, M. J. The small ubiquitin-like modifier-1 (SUMO-1) consensus sequence mediates Ubc9 binding and is essential for SUMO-1 modification. J. Biol. Chem. 276, 21664–21669 (2001)
Plemper, R. K. & Wolf, D. H. Retrograde protein translocation: ERADication of secretory proteins in health and disease. Trends Biochem. Sci. 24, 266–270 (1999)
Schillinger, W., Fiolet, J. W., Schlotthauer, K. & Hasenfuss, G. Relevance of Na+-Ca2+exchange in heart failure. Cardiovasc. Res. 57, 921–933 (2003)
Wang, J., Feng, X. H. & Schwartz, R. J. SUMO-1 modification activated GATA4-dependent cardiogenic gene activity. J. Biol. Chem. 279, 49091–49098 (2004)
Matsuzaki, K. et al. Serum response factor is modulated by the SUMO-1 conjugation system. Biochem. Biophys. Res. Commun. 306, 32–38 (2003)
Wang, J. & Schwartz, R. J. Sumoylation and regulation of cardiac gene expression. Circ. Res. 107, 19–29 (2010)
Brady, M. et al. Sp1 and Sp3 transcription factors are required for trans-activation of the human SERCA2 promoter in cardiomyocytes. Cardiovasc. Res. 60, 347–354 (2003)
Vlasblom, R. et al. Contractile arrest reveals calcium-dependent stimulation of SERCA2a mRNA expression in cultured ventricular cardiomyocytes. Cardiovasc. Res. 63, 537–544 (2004)
Drewett, V. et al. Serum response factor cleavage by caspases 3 and 7 linked to apoptosis in human BJAB cells. J. Biol. Chem. 276, 33444–33451 (2001)
Okura, T. et al. Protection against Fas/APO-1- and tumor necrosis factor-mediated cell death by a novel protein, sentrin. J. Immunol. 157, 4277–4281 (1996)
Baba, D. et al. Crystal structure of thymine DNA glycosylase conjugated to SUMO-1. Nature 435, 979–982 (2005)
Van Rechem, C. et al. Differential regulation of HIC1 target genes by CtBP and NuRD, via an acetylation/SUMOylation switch, in quiescent versus proliferating cells. Mol. Cell. Biol. 30, 4045–4059 (2010)
Choudhary, C. et al. Lysine acetylation targets protein complexes and co-regulates major cellular functions. Science 325, 834–840 (2009)
Kim, M. J., Chia, I. V. & Costantini, F. SUMOylation target sites at the C terminus protect axin from ubiquitination and confer protein stability. FASEB J. 22, 3785–3794 (2008)
Mooney, S. M., Grande, J. P., Salisbury, J. L. & Janknecht, R. Sumoylation of p68 and p72 RNA helicases affects protein stability and transactivation potential. Biochemistry 49, 1–10 (2010)
Ryu, K. Y., Garza, J. C., Lu, X. Y., Barsh, G. S. & Kopito, R. R. Hypothalamic neurodegeneration and adult-onset obesity in mice lacking the Ubb polyubiquitin gene. Proc. Natl Acad. Sci. USA 105, 4016–4021 (2008)
Hajjar, R. J., Schmidt, U., Kang, J. X., Matsui, T. & Rosenzweig, A. Adenoviral gene transfer of phospholamban in isolated rat cardiomyocytes. Rescue effects by concomitant gene transfer of sarcoplasmic reticulum Ca2+-ATPase. Circ. Res. 81, 145–153 (1997)
Lee, A. Y. et al. Identification of the degradome of Isp-1, a major intracellular serine protease of Bacillus subtilis, by two-dimensional gel electrophoresis and matrix- assisted laser desorption/ionization-time of flight analysis. Proteomics 4, 3437–3445 (2004)
Jeong, D. et al. PICOT inhibits cardiac hypertrophy and enhances ventricular function and cardiomyocyte contractility. Circ. Res. 99, 307–314 (2006)
Zolotukhin, S. et al. Recombinant adeno-associated virus purification using novel methods improves infectious titer and yield. Gene Ther. 6, 973–985 (1999)
Kizana, E., Cingolani, E. & Marban, E. Non-cell-autonomous effects of vector-expressed regulatory RNAs in mammalian heart cells. Gene Ther. 16, 1163–1168 (2009)
Tiscornia, G., Singer, O., Ikawa, M. & Verma, I. M. A general method for gene knockdown in mice by using lentiviral vectors expressing small interfering RNA. Proc. Natl Acad. Sci. USA 100, 1844–1848 (2003)
Hajjar, R. J. et al. Modulation of ventricular function through gene transfer in vivo . Proc. Natl Acad. Sci. USA 95, 5251–5256 (1998)
Pacher, P., Nagayama, T., Mukhopadhyay, P., Batkai, S. & Kass, D. A. Measurement of cardiac function using pressure-volume conductance catheter technique in mice and rats. Nature Protocols 3, 1422–1434 (2008)
Porterfield, J. E. et al. Dynamic correction for parallel conductance, GP, and gain factor, α, in invasive murine left ventricular volume measurements. J. Appl. Physiol. 107, 1693–1703 (2009)
Acknowledgements
This work is supported by NIH RO1 HL083156, HL080498, HL093183 and P20HL100396 (R.J.H.). W.J.P. is funded by Global Research Laboratory Program (M6-0605-00-0001) of the Korean Ministry of Science and Technology.
Author information
Authors and Affiliations
Contributions
C.K., A.L. and R.J.H. conceived the project and its design. C.K., A.L., D.J., J.G.O. and A.H.C. performed experiments and data analysis. E.K. aided in experimental design. C.K., A.L., W.J.P. and R.J.H. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
The file contains Supplementary Figures 1-11 with legends and Supplementary Tables 1-9. (PDF 9783 kb)
Rights and permissions
About this article
Cite this article
Kho, C., Lee, A., Jeong, D. et al. SUMO1-dependent modulation of SERCA2a in heart failure. Nature 477, 601–605 (2011). https://doi.org/10.1038/nature10407
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature10407
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.