Abstract
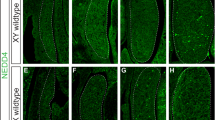
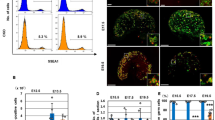
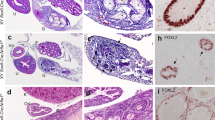
Sex in mammals is determined in the fetal gonad by the presence or absence of the Y chromosome gene Sry, which controls whether bipotential precursor cells differentiate into testicular Sertoli cells or ovarian granulosa cells1. This pivotal decision in a single gonadal cell type ultimately controls sexual differentiation throughout the body. Sex determination can be viewed as a battle for primacy in the fetal gonad between a male regulatory gene network in which Sry activates Sox9 and a female network involving WNT/β-catenin signalling2. In females the primary sex-determining decision is not final: loss of the FOXL2 transcription factor in adult granulosa cells can reprogram granulosa cells into Sertoli cells2. Here we show that sexual fate is also surprisingly labile in the testis: loss of the DMRT1 transcription factor3 in mouse Sertoli cells, even in adults, activates Foxl2 and reprograms Sertoli cells into granulosa cells. In this environment, theca cells form, oestrogen is produced and germ cells appear feminized. Thus Dmrt1 is essential to maintain mammalian testis determination, and competing regulatory networks maintain gonadal sex long after the fetal choice between male and female. Dmrt1 and Foxl2 are conserved throughout vertebrates4,5 and Dmrt1-related sexual regulators are conserved throughout metazoans3. Antagonism between Dmrt1 and Foxl2 for control of gonadal sex may therefore extend beyond mammals. Reprogramming due to loss of Dmrt1 also may help explain the aetiology of human syndromes linked to DMRT1, including disorders of sexual differentiation6 and testicular cancer7.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Accession codes
Primary accessions
Gene Expression Omnibus
Data deposits
mRNA expression profiling data have been deposited at the Gene Expression Omnibus under accession number GSE27261 and can be reviewed at http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?token5zlctbiugsamymtc&acc5GSE27261.
Change history
03 August 2011
Two typos in Fig. 3 and its legend have been corrected online but are incorrect in the print version.
References
Koopman, P., Gubbay, J., Vivian, N., Goodfellow, P. & Lovell-Badge, R. Male development of chromosomally female mice transgenic for Sry . Nature 351, 117–121 (1991)
Uhlenhaut, N. H. et al. Somatic sex reprogramming of adult ovaries to testes by FOXL2 ablation. Cell 139, 1130–1142 (2009)
Raymond, C. S. et al. Evidence for evolutionary conservation of sex-determining genes. Nature 391, 691–695 (1998)
Loffler, K. A., Zarkower, D. & Koopman, P. Etiology of ovarian failure in blepharophimosis ptosis epicanthus inversus syndrome: FOXL2 is a conserved, early-acting gene in vertebrate ovarian development. Endocrinology 144, 3237–3243 (2003)
Raymond, C. S., Kettlewell, J. R., Hirsch, B., Bardwell, V. J. & Zarkower, D. Expression of Dmrt1 in the genital ridge of mouse and chicken embryos suggests a role in vertebrate sexual development. Dev. Biol. 215, 208–220 (1999)
Tannour-Louet, M. et al. Identification of de novo copy number variants associated with human disorders of sexual development. PLoS ONE 5, e15392 (2010)
Turnbull, C. et al. Variants near DMRT1, TERT and ATF7IP are associated with testicular germ cell cancer. Nature Genet. 42, 604–607 (2010)
Yoshimoto, S. et al. A W-linked DM-domain gene, DM-W, participates in primary ovary development in Xenopus laevis . Proc. Natl Acad. Sci. USA 105, 2469–2474 (2008)
Smith, C. A. et al. The avian Z-linked gene DMRT1 is required for male sex determination in the chicken. Nature 461, 267–271 (2009)
Matsuda, M. et al. DMY is a Y-specific DM-domain gene required for male development in the medaka fish. Nature 417, 559–563 (2002)
Kim, S., Bardwell, V. J. & Zarkower, D. Cell type-autonomous and non-autonomous requirements for Dmrt1 in postnatal testis differentiation. Dev. Biol. 307, 314–327 (2007)
Matson, C. K. et al. The mammalian doublesex homolog DMRT1 is a transcriptional gatekeeper that controls the mitosis versus meiosis decision in male germ cells. Dev. Cell 19, 612–624 (2010)
Krentz, A. D. et al. The DM domain protein DMRT1 is a dose-sensitive regulator of fetal germ cell proliferation and pluripotency. Proc. Natl Acad. Sci. USA 106, 22323–22328 (2009)
Raymond, C. S., Murphy, M. W., O'Sullivan, M. G., Bardwell, V. J. & Zarkower, D. Dmrt1, a gene related to worm and fly sexual regulators, is required for mammalian testis differentiation. Genes Dev. 14, 2587–2595 (2000)
Schmidt, D. et al. The murine winged-helix transcription factor Foxl2 is required for granulosa cell differentiation and ovary maintenance. Development 131, 933–942 (2004)
Uda, M. et al. Foxl2 disruption causes mouse ovarian failure by pervasive blockage of follicle development. Hum. Mol. Genet. 13, 1171–1181 (2004)
Fahrioglu, U., Murphy, M. W., Zarkower, D. & Bardwell, V. J. mRNA expression analysis and the molecular basis of neonatal testis defects in Dmrt1 mutant mice. Sex. Dev. 1, 42–58 (2007)
Murphy, M. W. et al. Genome-wide analysis of DNA binding and transcriptional regulation by the mammalian Doublesex homolog DMRT1 in the juvenile testis. Proc. Natl Acad. Sci. USA 107, 13360–13365 (2010)
Cocquet, J., Pannetier, M., Fellous, M. & Veitia, R. A. Sense and antisense Foxl2 transcripts in mouse. Genomics 85, 531–541 (2005)
Duggavathi, R. et al. Liver receptor homolog 1 is essential for ovulation. Genes Dev. 22, 1871–1876 (2008)
Orisaka, M., Tajima, K., Tsang, B. K. & Kotsuji, F. Oocyte-granulosa-theca cell interactions during preantral follicular development. J. Ovarian Res. 2, 9 (2009)
Murphy, M. W., Zarkower, D. & Bardwell, V. J. Vertebrate DM domain proteins bind similar DNA sequences and can heterodimerize on DNA. BMC Mol. Biol. 8, 58 (2007)
Chaboissier, M. C. et al. Functional analysis of Sox8 and Sox9 during sex determination in the mouse. Development 131, 1891–1901 (2004)
Barrionuevo, F. et al. Testis cord differentiation after the sex determination stage is independent of Sox9 but fails in the combined absence of Sox9 and Sox8 . Dev. Biol. 327, 301–312 (2009)
Chang, H. et al. Wt1 negatively regulates β-catenin signaling during testis development. Development 135, 1875–1885 (2008)
Vainio, S., Heikkila, M., Kispert, A., Chin, N. & McMahon, A. P. Female development in mammals is regulated by Wnt-4 signalling. Nature 397, 405–409 (1999)
Parma, P. et al. R-spondin1 is essential in sex determination, skin differentiation and malignancy. Nature Genet. 38, 1304–1309 (2006)
Wu, J., Irazarry, R. A., Gentleman, R., Martinez-Murillo, F. & Spencer, F. A model-based background adjustment for oligonucleotide expression arrays. J. Am. Stat. Assoc. 99, 909–917 (2004)
Edgar, R., Domrachev, M. & Lash, A. E. Gene Expression Omnibus: NCBI gene expression and hybridization array data repository. Nucleic Acids Res. 30, 207–210 (2002)
de Hoon, M. J., Imoto, S., Nolan, J. & Miyano, S. Open source clustering software. Bioinformatics 20, 1453–1454 (2004)
Saldanha, A. J. Java Treeview—extensible visualization of microarray data. Bioinformatics 20, 3246–3248 (2004)
Acknowledgements
We thank M. Treier for helpful discussion, K. Hatzi, A. Minkina, A. Peterson, the University of Minnesota Mouse Genetics Laboratory and C. Small for technical assistance, J. Dean, R. Veitia and K.-i. Morohashi for antibodies, D. Greenstein and A. M. Weber-Main for comments on the manuscript, C. Manivel for histology expertise, M. Steffes and D. Gabrielson for oestradiol analysis, and the University of Minnesota Supercomputing Institute for computational resources. This work was funded by the NIH (GM59152), the Minnesota Medical Foundation, and a predoctoral fellowship from the NSF (to C.K.M.).
Author information
Authors and Affiliations
Contributions
C.K.M. performed mouse breeding and analysis of protein and mRNA expression; M.W.M. performed ChIP analysis; A.L.S. performed bioinformatic analysis; C.K.M., D.Z. and V.J.B. designed the study, analysed data, and wrote the paper; M.D.G. provided mRNA profiling expertise; all authors discussed the results and edited the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
The file contains Supplementary Methods, Supplementary Tables 1-4, Supplementary Figures 1-11 with legends and additional references. (PDF 6402 kb)
Rights and permissions
About this article
Cite this article
Matson, C., Murphy, M., Sarver, A. et al. DMRT1 prevents female reprogramming in the postnatal mammalian testis. Nature 476, 101–104 (2011). https://doi.org/10.1038/nature10239
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature10239
This article is cited by
-
Testicular macrophages are recruited during a narrow fetal time window and promote organ-specific developmental functions
Nature Communications (2023)
-
Enhancement of zebrafish sperm production via a large body-sized surrogate with germ cell transplantation
Communications Biology (2023)
-
PRC1 suppresses a female gene regulatory network to ensure testicular differentiation
Cell Death & Disease (2023)
-
Evolution of sex determination in crustaceans
Marine Life Science & Technology (2023)
-
Associations between testicular development and fetal size in the pig
Journal of Animal Science and Biotechnology (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.