Abstract
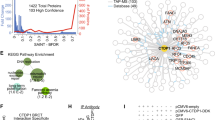
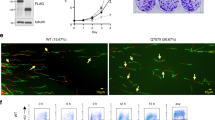
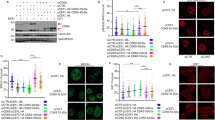
Cyclin D1 is a component of the core cell cycle machinery1. Abnormally high levels of cyclin D1 are detected in many human cancer types2. To elucidate the molecular functions of cyclin D1 in human cancers, we performed a proteomic screen for cyclin D1 protein partners in several types of human tumours. Analyses of cyclin D1 interactors revealed a network of DNA repair proteins, including RAD51, a recombinase that drives the homologous recombination process3. We found that cyclin D1 directly binds RAD51, and that cyclin D1–RAD51 interaction is induced by radiation. Like RAD51, cyclin D1 is recruited to DNA damage sites in a BRCA2-dependent fashion. Reduction of cyclin D1 levels in human cancer cells impaired recruitment of RAD51 to damaged DNA, impeded the homologous recombination-mediated DNA repair, and increased sensitivity of cells to radiation in vitro and in vivo. This effect was seen in cancer cells lacking the retinoblastoma protein, which do not require D-cyclins for proliferation4,5. These findings reveal an unexpected function of a core cell cycle protein in DNA repair and suggest that targeting cyclin D1 may be beneficial also in retinoblastoma-negative cancers which are currently thought to be unaffected by cyclin D1 inhibition.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Sherr, C. J. & Roberts, J. M. Living with or without cyclins and cyclin-dependent kinases. Genes Dev. 18, 2699–2711 (2004)
Deshpande, A., Sicinski, P. & Hinds, P. W. Cyclins and cdks in development and cancer: a perspective. Oncogene 24, 2909–2915 (2005)
Baumann, P. & West, S. C. Role of the human RAD51 protein in homologous recombination and double-stranded-break repair. Trends Biochem. Sci. 23, 247–251 (1998)
Bates, S. et al. Absence of cyclin D/cdk complexes in cells lacking functional retinoblastoma protein. Oncogene 9, 1633–1640 (1994)
Lukas, J. et al. Retinoblastoma-protein-dependent cell-cycle inhibition by the tumour suppressor p16. Nature 375, 503–506 (1995)
Ek, S., Ortega, E. & Borrebaeck, C. A. K. Transcriptional profiling and assessment of cell lines as in vitro models for mantle cell lymphoma. Leuk. Res. 29, 205–213 (2005)
Lin, D. I. et al. Phosphorylation-dependent ubiquitination of cyclin D1 by the SCFEBX4-αB crystallin complex. Mol. Cell 24, 355–366 (2006)
Hosokawa, Y. & Arnold, A. Mechanism of cyclin D1 (CCND1, PRAD1) overexpression in human cancer cells: analysis of allele-specific expression. Genes Chromosom. Cancer 22, 66–71 (1998)
Bartkova, J. et al. Abnormal patterns of D-type cyclin expression and G1 regulation in human head and neck cancer. Cancer Res. 55, 949–956 (1995)
Nakatani, Y. & Ogryzko, V. Immunoaffinity purification of mammalian protein complexes. Methods Enzymol. 370, 430–444 (2003)
Kozar, K. et al. Mouse development and cell proliferation in the absence of D-cyclins. Cell 118, 477–491 (2004)
Toogood, P. L. et al. Discovery of a potent and selective inhibitor of cyclin-dependent kinase 4/6. J. Med. Chem. 48, 2388–2406 (2005)
Hinds, P. W., Dowdy, S. F., Eaton, E. N., Arnold, A. & Weinberg, R. A. Function of a human cyclin gene as an oncogene. Proc. Natl Acad. Sci. USA 91, 709–713 (1994)
Landis, M. W., Pawlyk, B. S., Li, T., Sicinski, P. & Hinds, P. W. Cyclin D1-dependent kinase activity in murine development and mammary tumorigenesis. Cancer Cell 9, 13–22 (2006)
Ostling, O. & Johanson, K. J. Microelectrophoretic study of radiation-induced DNA damages in individual mammalian cells. Biochem. Biophys. Res. Commun. 123, 291–298 (1984)
Baumann, P., Benson, F. E. & West, S. C. Human Rad51 protein promotes ATP-dependent homologous pairing and strand transfer reactions in vitro . Cell 87, 757–766 (1996)
Pierce, A. J., Johnson, R. D., Thompson, L. H. & Jasin, M. XRCC3 promotes homology-directed repair of DNA damage in mammalian cells. Genes Dev. 13, 2633–2638 (1999)
Lord, C. J. & Ashworth, A. Targeted therapy for cancer using PARP inhibitors. Curr. Opin. Pharmacol. 8, 363–369 (2008)
Agami, R. & Bernards, R. Distinct initiation and maintenance mechanisms cooperate to induce G1 cell cycle arrest in response to DNA damage. Cell 102, 55–66 (2000)
Pontano, L. L. et al. Genotoxic stress-induced cyclin D1 phosphorylation and proteolysis are required for genomic stability. Mol. Cell. Biol. 28, 7245–7258 (2008)
Coco Martin, J. M., Balkenende, A., Verschoor, T., Lallemand, F. & Michalides, R. Cyclin D1 overexpression enhances radiation-induced apoptosis and radiosensitivity in a breast tumor cell line. Cancer Res. 59, 1134–1140 (1999)
Sugawara, N., Wang, X. & Haber, J. E. In vivo roles of Rad52, Rad54, and Rad55 proteins in Rad51-mediated recombination. Mol. Cell 12, 209–219 (2003)
Wolner, B., van Komen, S., Sung, P. & Peterson, C. L. Recruitment of the recombinational repair machinery to a DNA double-strand break in yeast. Mol. Cell 12, 221–232 (2003)
Rodrigue, A. et al. Interplay between human DNA repair proteins at a unique double-strand break in vivo . EMBO J. 25, 222–231 (2006)
West, S. C. Molecular views of recombination proteins and their control. Nature Rev. Mol. Cell Biol. 4, 435–445 (2003)
Lee, M., Daniels, M. J. & Venkitaraman, A. R. Phosphorylation of BRCA2 by the Polo-like kinase Plk1 is regulated by DNA damage and mitotic progression. Oncogene 23, 865–872 (2004)
Li, Z. et al. Alternative cyclin D1 splice forms differentially regulate the DNA damage response. Cancer Res. 70, 8802–8811 (2010)
Beroukhim, R. et al. The landscape of somatic copy-number alteration across human cancers. Nature 463, 899–905 (2010)
Bienvenu, F. et al. Transcriptional role of cyclin D1 in development revealed by a genetic-proteomic screen. Nature 463, 374–378 (2010)
Acknowledgements
We thank the members of the Sicinski lab for help and advice, N. Li for help with initial experiments, S Panyim and Y. Geng for discussions and reading the manuscript, P. Nakatani for pOZ-FH-N construct, M. Jasin for DR-GFP system, D. Bulavin and E. Appella for anti-phospho-CDC25A antibodies, A. Smogorzewska for anti-FANCI antibody, A. Venkitaraman and M. Lee for GST–BRCA2 fragments, and DFCI Confocal and Light Microscopy Core Facility for assisting with confocal microscopy. This work was supported by R01 CA083688, P01 CA080111 and P01 CA109901 grants from NIH (to P.S.). S.J. is supported by The Thailand Research Fund MRG5280248, W.M. by Foundation for Polish Science, Y.E.W. through the CCCB and the Dana-Farber Strategic Plan Initiative, A.B.C. and T.A.K. by Project Z01 ES065089 (to T.A.K.) from the Division of Intramural Research of the NIH, NIEHS.
Author information
Authors and Affiliations
Contributions
S.J. and P.S. designed the study, analysed the data and wrote the manuscript. S.J. performed most of the experiments with help from collaborators. Y.H. and D.M.L. contributed to DNA repair analyses. W.M. contributed in vitro protein binding analyses. J.E.E. and T.G. performed mass spectrometry; J.E.E. analysed mass spec data with S.P.G. L.B., F.B., A.Z., T.v.H helped with experiments. Y.E.W. M.C. and J.Q. performed computational analyses of interactors. A.B.C. and T.A.K. contributed DNA mismatch repair analyses. B.X. helped with BRCA2 analyses.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Figures
This file contains Supplementary Figures 1-28 with legends. (PDF 3085 kb)
Supplementary Information
This file contains Supplementary Methods and additional references. (PDF 972 kb)
Supplementary Table 1
This table contains a list of peptides identified in five human cancer cell lines. (XLS 7218 kb)
Supplementary Table 2
This table contains a list of proteins identified in five human cancer cell lines. (XLS 1191 kb)
Supplementary Table 3
This table contains a list of proteins identified in five human cancer cell lines. (XLS 155 kb)
Supplementary Table 4
This table contains a list of proteins identified in five human cancer cell lines. (XLS 60 kb)
Supplementary Table 5
This table contains a summary of proteomic data for proteins mentioned in the text. (XLS 64 kb)
Supplementary Table 6
This table contains the molecular function biological process analyses of cyclin D1 interacting proteins. (XLS 184 kb)
Rights and permissions
About this article
Cite this article
Jirawatnotai, S., Hu, Y., Michowski, W. et al. A function for cyclin D1 in DNA repair uncovered by protein interactome analyses in human cancers. Nature 474, 230–234 (2011). https://doi.org/10.1038/nature10155
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature10155
This article is cited by
-
Recent advances in melittin-based nanoparticles for antitumor treatment: from mechanisms to targeted delivery strategies
Journal of Nanobiotechnology (2023)
-
A genome-wide CRISPR-Cas9 knockout screen identifies novel PARP inhibitor resistance genes in prostate cancer
Oncogene (2022)
-
NKX6-1 mediates cancer stem-like properties and regulates sonic hedgehog signaling in leiomyosarcoma
Journal of Biomedical Science (2021)
-
Inhibition of Kpnβ1 mediated nuclear import enhances cisplatin chemosensitivity in cervical cancer
BMC Cancer (2021)
-
Apolipoprotein C1 stimulates the malignant process of renal cell carcinoma via the Wnt3a signaling
Cancer Cell International (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.