Abstract
Arising from F. Huang, P. et al. Nature 464, 396–400 (2010)10.1038/nature08840; Huang et al. reply
It was recently shown that relatively large (compared to analytical precision) steady state thermal isotope fractionations are produced in silicate melts whenever temperature differences are maintained for a sufficiently long time1,2. Huang et al.3 reported new data on thermal isotopic fractionation of magnesium, calcium, and iron in silicate liquids, and claimed (1) that thermal isotopic fractionations in silicate liquids are independent of composition and temperature, and (2) that their “results lead to a simple and robust framework for characterizing isotope fractionations by thermal diffusion in natural and synthetic systems”. Here I consider whether the data and arguments presented by Huang et al.3 support their claims. In summary, I caution against assuming (on the basis of the data presented by Huang et al.3) that the thermal isotopic fractionations are independent of temperature and composition, or that a framework of the type claimed has been found.
Similar content being viewed by others
Main
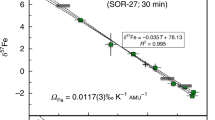
Huang et al.3 reported new data on thermal isotopic fractionation of magnesium, calcium and iron in silicate liquids that extend the earlier results1,2 to a larger temperature range and to compositions other than basalt. The magnesium isotopic composition versus temperature reported by Huang et al.3 for molten andesite and basalt are shown in Fig. 1. The local slope in such a plot determines the thermal-diffusion isotopic sensitivity Ω, defined as the magnitude of isotopic fractionation per temperature offset, and expressed in units of ‰ °C−1 amu−1 (here amu indicates atomic mass unit). If Ω is independent of temperature, the data would fall on straight lines, which is clearly not the case for the andesite experiments (squares in Fig. 1), where the slope of the isotopic fractionations versus temperature shows that ΩMg varies from about 0.05 ‰ °C−1 amu−1 for T > 1,450 °C to about 0.02 ‰ °C−1 amu−1 for T < 1,450 °C. Despite this, Huang et al.3 imply in their figure 1 that ΩMg = 0.03 ‰ °C−1 amu−1 with an uncertainty of only ±10%. The iron isotope data reported by Huang et al.3 also show large variations of ΩFe with temperature.
Data are taken from table 1 in Huang et al.3. Shown are values for molten Mount Hood andesite (squares for data from a 46-h experiment, circles for data from a 168-h experiment), and for molten mid-ocean-ridge basalt (diamonds for data from a 264-h experiment). The isotopic fractionations are given as  . The figure shows that in the case of andesite, ΩMg is not constant, but rather decreases by a factor of more than two between T > 1,500 °C and T < 1,500°C. The data for basalt are too few to support a conclusion to the effect that ΩMg is independent of composition.
. The figure shows that in the case of andesite, ΩMg is not constant, but rather decreases by a factor of more than two between T > 1,500 °C and T < 1,500°C. The data for basalt are too few to support a conclusion to the effect that ΩMg is independent of composition.
Huang et al.3 claim to have cast their experimental observations in a theoretical framework. This ‘theoretical framework’ is simply a parameterization of an assumed, not theoretically derived, functional form for the mass dependence of a quantity they call ΔST, which, like Ω, is proportional to the slope of the thermal isotope fractionations versus temperature. The assumed expression for ΔST is  , where X and Y are the mass of two isotopes of an element of valence Z and ionic radius a. The three quantities c, α and β were adjusted such that the calculated ΔST reproduces three values of ΔST taken from their experiments. Leaving aside the issues of what specific value of ΔST to choose, given its variation with temperature, which is proportional to the slope of δ26Mg versus temperature shown in Fig. 1, or that all the elements considered have the same Z, using a parameterization with three free parameters to fit three data does not constrain and validate the functional form of the parameterization. One can, however, test the proposed parameterization by calculating ΔST for all the major elements of basalt (magnesium, calcium, iron, silicon and oxygen) and comparing these to the experimental data reported by Richter et al.2. As might be expected, the parameterization works reasonably well in reproducing the measured ΔST for magnesium, calcium and iron; however, when applied to oxygen and silicon, the calculated values and the measured values differ by more than a factor two for oxygen and more than a factor of ten for silicon.
, where X and Y are the mass of two isotopes of an element of valence Z and ionic radius a. The three quantities c, α and β were adjusted such that the calculated ΔST reproduces three values of ΔST taken from their experiments. Leaving aside the issues of what specific value of ΔST to choose, given its variation with temperature, which is proportional to the slope of δ26Mg versus temperature shown in Fig. 1, or that all the elements considered have the same Z, using a parameterization with three free parameters to fit three data does not constrain and validate the functional form of the parameterization. One can, however, test the proposed parameterization by calculating ΔST for all the major elements of basalt (magnesium, calcium, iron, silicon and oxygen) and comparing these to the experimental data reported by Richter et al.2. As might be expected, the parameterization works reasonably well in reproducing the measured ΔST for magnesium, calcium and iron; however, when applied to oxygen and silicon, the calculated values and the measured values differ by more than a factor two for oxygen and more than a factor of ten for silicon.
I caution against assuming (on the basis of the data presented by Huang et al.3) that thermal isotope fractionations in silicate liquids are independent of temperature and composition or that they have found “a simple and robust framework for characterizing isotope fractionations by thermal diffusion in natural and synthetic systems”.
References
Richter, F. M., Watson, E. B., Mendybaev, R. A., Teng, F.-Z. & Janney, P. E. Magnesium isotope fractionation in silicate melts by chemical and thermal diffusion. Geochim. Cosmochim. Acta 72, 206–220 (2008)
Richter, F. M. et al. Isotope fractionation of the major elements of molten basalt by chemical and thermal diffusion. Geochim. Cosmochim. Acta 73, 4250–4263 (2009)
Huang, F. et al. Isotope fractionation in silicate melts by thermal diffusion. Nature 464, 396–400 (2010)
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Competing financial interests: declared none.
PowerPoint slides
Rights and permissions
About this article
Cite this article
Richter, F. Isotope fractionation in silicate melts by thermal diffusion. Nature 472, E1 (2011). https://doi.org/10.1038/nature09954
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature09954
This article is cited by
-
Huang et al. reply
Nature (2011)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.