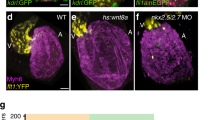
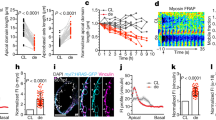
Abstract
Electrical gradients are critical for many biological processes, including the normal function of excitable tissues, left–right patterning, organogenesis and wound healing1,2,3,4. The fundamental mechanisms that regulate the establishment and maintenance of such electrical polarities are poorly understood. Here we identify a gradient of electrical coupling across the developing ventricular myocardium using high-speed optical mapping of transmembrane potentials and calcium concentrations in the zebrafish heart. We excluded a role for differences in cellular excitability, connexin localization, tissue geometry and mechanical inputs, but in contrast we were able to demonstrate that non-canonical Wnt11 signals are required for the genesis of this myocardial electrical gradient. Although the traditional planar cell polarity pathway is not involved, we obtained evidence that Wnt11 acts to set up this gradient of electrical coupling through effects on transmembrane Ca2+ conductance mediated by the L-type calcium channel. These data reveal a previously unrecognized role for Wnt/Ca2+ signalling in establishing an electrical gradient in the plane of the developing cardiac epithelium through modulation of ion-channel function. The regulation of cellular coupling through such mechanisms may be a general property of non-canonical Wnt signals.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Costantini, D. L. et al. The homeodomain transcription factor Irx5 establishes the mouse cardiac ventricular repolarization gradient. Cell 123, 347–358 (2005)
Yao, L., McCaig, C. D. & Zhao, M. Electrical signals polarize neuronal organelles, direct neuron migration, and orient cell division. Hippocampus 19, 855–868 (2009)
Adams, D. S. et al. Early, H+-V-ATPase-dependent proton flux is necessary for consistent left-right patterning of non-mammalian vertebrates. Development 133, 1657–1671 (2006)
Zhao, M. et al. Electrical signals control wound healing through phosphatidylinositol-3-OH kinase-gamma and PTEN. Nature 442, 457–460 (2006)
Watanabe, T., Delbridge, L. M., Bustamante, J. O. & McDonald, T. F. Heterogeneity of the action potential in isolated rat ventricular myocytes and tissue. Circ. Res. 52, 280–290 (1983)
Milan, D. J., Giokas, A. C., Serluca, F. C., Peterson, R. T. & MacRae, C. A. Notch1b and neuregulin are required for specification of central cardiac conduction tissue. Development 133, 1125–1132 (2006)
Chi, N. C. et al. Genetic and physiologic dissection of the vertebrate cardiac conduction system. PLoS Biol. 6, e109 (2008)
Hoyt, R. H., Cohen, M. L. & Saffitz, J. E. Distribution and three-dimensional structure of intercellular junctions in canine myocardium. Circ. Res. 64, 563–574 (1989)
Chung, C. Y., Bien, H. & Entcheva, E. The role of cardiac tissue alignment in modulating electrical function. J. Cardiovasc. Electrophysiol. 18, 1323–1329 (2007)
Auman, H. J. et al. Functional modulation of cardiac form through regionally confined cell shape changes. PLoS Biol. 5, e53 (2007)
Sehnert, A. J. et al. Cardiac troponin T is essential in sarcomere assembly and cardiac contractility. Nature Genet. 31, 106–110 (2002)
Etard, C. et al. The UCS factor Steif/Unc-45b interacts with the heat shock protein Hsp90a during myofibrillogenesis. Dev. Biol. 308, 133–143 (2007)
Cohen, E. D., Tian, Y. & Morrisey, E. E. Wnt signaling: an essential regulator of cardiovascular differentiation, morphogenesis and progenitor self-renewal. Development 135, 789–798 (2008)
Eisenberg, C. A. & Eisenberg, L. M. WNT11 promotes cardiac tissue formation of early mesoderm. Dev. Dyn. 216, 45–58 (1999)
Pandur, P., Lasche, M., Eisenberg, L. M. & Kuhl, M. Wnt-11 activation of a non-canonical Wnt signalling pathway is required for cardiogenesis. Nature 418, 636–641 (2002)
Zhou, W. et al. Modulation of morphogenesis by noncanonical Wnt signaling requires ATF/CREB family-mediated transcriptional activation of TGFbeta2. Nature Genet. 39, 1225–1234 (2007)
Flaherty, M. P. & Dawn, B. Noncanonical Wnt11 signaling and cardiomyogenic differentiation. Trends Cardiovasc. Med. 18, 260–268 (2008)
Thisse, C. & Thisse, B. High throughput expression analysis of ZF-models consortium clones. ZFIN Direct Data Submission ZDB-PUB-051025-1 〈http://zfin.org/cgi-bin/webdriver?MIval=aa-pubview2apg&OID=ZDB-PUB-051025-1〉 (2005)
Robu, M. E. et al. p53 activation by knockdown technologies. PLoS Genet. 3, e78 (2007)
Yao, S. et al. Pnas4 is a novel regulator for convergence and extension during vertebrate gastrulation. FEBS Lett. 582, 2325–2332 (2008)
Heisenberg, C. P. et al. Silberblick/Wnt11 mediates convergent extension movements during zebrafish gastrulation. Nature 405, 76–81 (2000)
Veeman, M. T., Axelrod, J. D. & Moon, R. T. A second canon. Dev. Cell 5, 367–377 (2003)
Jessen, J. R. et al. Zebrafish trilobite identifies new roles for Strabismus in gastrulation and neuronal movements. Nature Cell Biol. 4, 610–615 (2002)
Veeman, M. T., Slusarski, D. C., Kaykas, A., Louie, S. H. & Moon, R. T. Zebrafish prickle, a modulator of noncanonical Wnt/Fz signaling, regulates gastrulation movements. Curr. Biol. 13, 680–685 (2003)
Slusarski, D. C., Corces, V. G. & Moon, R. T. Interaction of Wnt and a Frizzled homologue triggers G-protein-linked phosphatidylinositol signalling. Nature 390, 410–413 (1997)
Bers, D. M. Calcium cycling and signaling in cardiac myocytes. Annu. Rev. Physiol. 70, 23–49 (2008)
Stainier, D. Y. et al. Mutations affecting the formation and function of the cardiovascular system in the zebrafish embryo. Development 123, 285–292 (1996)
Witzel, S., Zimyanin, V., Carreira-Barbosa, F., Tada, M. & Heisenberg, C. P. Wnt11 controls cell contact persistence by local accumulation of Frizzled 7 at the plasma membrane. J. Cell Biol. 175, 791–802 (2006)
Rottbauer, W. et al. Reptin and pontin antagonistically regulate heart growth in zebrafish embryos. Cell 111, 661–672 (2002)
Angers, S. & Moon, R. T. Proximal events in Wnt signal transduction. Nature Rev. Mol. Cell Biol. 10, 468–477 (2009)
Loew, L. M. et al. A naphtyl analog of the aminostyryl pyridinium class of potentiometric membrane dyes shows consistent sensitivity in a variety of tissue, cell, and model membrane preparations. J. Membr. Biol. 130, 1–10 (1992)
Fast, V. G. & Kleber, A. G. Microscopic conduction in cultured strands of neonatal rat heart cells measured with voltage-sensitive dyes. Circ. Res. 73, 914–925 (1993)
Rohr, S. & Salzberg, B. M. Multiple site optical recording of transmembrane voltage (MSORTV) in patterned growth heart cell cultures: assessing electrical behaviour, with microsecond resolution, on a cellular and subcellular scale. Biophys. J. 67, 1301–1315 (1994)
Girouard, S. D., Laurita, K. R. & Rosenbaum, D. S. Unique properties of cardiac action potentials recorded with voltage-sensitive dyes. J. Cardiovasc. Electrophysiol. 7, 1024–1038 (1996)
Rohr, S. & Kucera, J. P. Optical recording system based on a fiber optic image conduit: assessment of microscopic activation patterns in cardiac tissue. Biophys. J. 75, 1062–1075 (1998)
Windisch, H. in Optical Mapping of Cardiac Excitation and Arrhythmias (eds Rosenbaum, D. S. & Jalife, J.) 97–112 (Futura, 2001)
Milan, D. J., Jones, I. L., Ellinor, P. T. & MacRae, C. A. In vivo recording of adult zebrafish electrocardiogram and assessment of drug-induced QT prolongation. Am. J. Physiol. Heart Circ. Physiol. 291, H269–H273 (2006)
Kikuchi, K. et al. Primary contribution to zebrafish heart regeneration by gata4+ cardiomyocytes. Nature 464, 601–605 (2010)
Fast, V. G. & Kleber, A. G. Cardiac tissue geometry as a determinant of unidirectional conduction block: assessment of microscopic excitation spread by optical mapping in patterned cell cultures and in a computer model. Cardiovasc. Res. 29, 697–707 (1995)
Bayly, P. V. et al. Estimation of conduction velocity vector fields from epicardial mapping data. IEEE Trans. Biomed. Eng. 45, 563–571 (1998)
Eloff, B. C. et al. High resolution optical mapping reveals conduction slowing in connexin43 deficient mice. Cardiovasc. Res. 51, 681–690 (2001)
Mills, R. W., Narayan, S. M. & McCulloch, A. D. Mechanisms of conduction slowing during myocardial stretch by ventricular volume loading in the rabbit. Am. J. Physiol. Heart Circ. Physiol. 295, H1270–H1278 (2008)
Acknowledgements
We thank C. P. Heisenberg, L. Solnica-Krezel and J. Mably for the gift of reagents. We thank R. Peterson and I. Drummond for comments on the manuscript. D.P. is supported by a fellowship from the HFSP. A.A.W. was supported by an NIH training award to the CVRC at MGH. C.A.M. is supported by the March of Dimes and the NIH.
Author information
Authors and Affiliations
Contributions
D.P. designed and performed the genetic, immunohistochemical and optical mapping experiments; A.A.W. devised techniques for optical voltage mapping and ratiometric Ca2+ imaging, developed analysis software, and designed and performed the corresponding experiments; C.A.M. designed the experimental strategy. D.P., A.A.W. and C.A.M. analysed the data and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Figures
This file contains Supplementary Figures 1-7 with legends. (PDF 443 kb)
Supplementary Movie 1
This movie shows the action potential propagation in wild type linear heart tube at 24 hpf. Action potential propagation is slow and homogeneous. (MOV 9122 kb)
Supplementary Movie 2
This movie shows the action potential propagation in wild type heart at 72 hpf, after looping is complete. (MOV 3892 kb)
Supplementary Movie 3
This movie shows the action potential propagation in a heart from Wnt11 morphant embryos at 72 hpf. (MOV 2855 kb)
Rights and permissions
About this article
Cite this article
Panáková, D., Werdich, A. & MacRae, C. Wnt11 patterns a myocardial electrical gradient through regulation of the L-type Ca2+ channel. Nature 466, 874–878 (2010). https://doi.org/10.1038/nature09249
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature09249
This article is cited by
-
Outlining cardiac ion channel protein interactors and their signature in the human electrocardiogram
Nature Cardiovascular Research (2023)
-
A bioelectrical phase transition patterns the first vertebrate heartbeats
Nature (2023)
-
Modeling of large-scale hoxbb cluster deletions in zebrafish uncovers a role for segmentation pathways in atrioventricular boundary specification
Cellular and Molecular Life Sciences (2023)
-
Genome-wide association analyses identify new Brugada syndrome risk loci and highlight a new mechanism of sodium channel regulation in disease susceptibility
Nature Genetics (2022)
-
Planar cell polarity signalling coordinates heart tube remodelling through tissue-scale polarisation of actomyosin activity
Nature Communications (2018)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.