Abstract
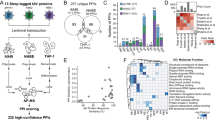
Influenza A virus is an RNA virus that encodes up to 11 proteins and this small coding capacity demands that the virus use the host cellular machinery for many aspects of its life cycle1. Knowledge of these host cell requirements not only informs us of the molecular pathways exploited by the virus but also provides further targets that could be pursued for antiviral drug development. Here we use an integrative systems approach, based on genome-wide RNA interference screening, to identify 295 cellular cofactors required for early-stage influenza virus replication. Within this group, those involved in kinase-regulated signalling, ubiquitination and phosphatase activity are the most highly enriched, and 181 factors assemble into a highly significant host–pathogen interaction network. Moreover, 219 of the 295 factors were confirmed to be required for efficient wild-type influenza virus growth, and further analysis of a subset of genes showed 23 factors necessary for viral entry, including members of the vacuolar ATPase (vATPase) and COPI-protein families, fibroblast growth factor receptor (FGFR) proteins, and glycogen synthase kinase 3 (GSK3)-β. Furthermore, 10 proteins were confirmed to be involved in post-entry steps of influenza virus replication. These include nuclear import components, proteases, and the calcium/calmodulin-dependent protein kinase (CaM kinase) IIβ (CAMK2B). Notably, growth of swine-origin H1N1 influenza virus is also dependent on the identified host factors, and we show that small molecule inhibitors of several factors, including vATPase and CAMK2B, antagonize influenza virus replication.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Palese, P. & Shaw, M. L. in Fields Virology 5th edn, Vol. 2 (eds D. M. Knipe & P. M. Howley) Ch. 47 1647–1689 (Lippincott Williams & Wilkins, 2007)
Wang, T. T. & Palese, P. Unraveling the mystery of swine influenza virus. Cell 137, 983–985 (2009)
De Clercq, E. Antiviral agents active against influenza A viruses. Nature Rev. Drug Discov. 5, 1015–1025 (2006)
Layne, S. P., Monto, A. S. & Taubenberger, J. K. Pandemic influenza: an inconvenient mutation. Science 323, 1560–1561 (2009)
Brass, A. L. et al. Identification of host proteins required for HIV infection through a functional genomic screen. Science 319, 921–926 (2008)
König, R. et al. Global analysis of host-pathogen interactions that regulate early-stage HIV-1 replication. Cell 135, 49–60 (2008)
Zhou, H. et al. Genome-scale RNAi screen for host factors required for HIV replication. Cell Host Microbe 4, 495–504 (2008)
Krishnan, M. N. et al. RNA interference screen for human genes associated with West Nile virus infection. Nature 455, 242–245 (2008)
Li, Q. et al. A genome-wide genetic screen for host factors required for hepatitis C virus propagation. Proc. Natl Acad. Sci. USA 10.1073/pnas.0907439106 (27 August 2009)
Tai, A. W. et al. A functional genomic screen identifies cellular cofactors of hepatitis C virus replication. Cell Host Microbe 5, 298–307 (2009)
Sessions, O. M. et al. Discovery of insect and human dengue virus host factors. Nature 458, 1047–1050 (2009)
Hao, L. et al. Drosophila RNAi screen identifies host genes important for influenza virus replication. Nature 454, 890–893 (2008)
Marsh, G. A., Hatami, R. & Palese, P. Specific residues of the influenza A virus hemagglutinin viral RNA are important for efficient packaging into budding virions. J. Virol. 81, 9727–9736 (2007)
König, R. et al. A probability-based approach for the analysis of large-scale RNAi screens. Nature Methods 4, 847–849 (2007)
Sato, S., Fujita, N. & Tsuruo, T. Modulation of Akt kinase activity by binding to Hsp90. Proc. Natl Acad. Sci. USA 97, 10832–10837 (2000)
Sarbassov, D. D., Guertin, D. A., Ali, S. M. & Sabatini, D. M. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science 307, 1098–1101 (2005)
Venkatesan, K. et al. An empirical framework for binary interactome mapping. Nature Methods 6, 83–90 (2009)
Beer, C., Andersen, D. S., Rojek, A. & Pedersen, L. Caveola-dependent endocytic entry of amphotropic murine leukemia virus. J. Virol. 79, 10776–10787 (2005)
McClure, M. O., Sommerfelt, M. A., Marsh, M. & Weiss, R. A. The pH independence of mammalian retrovirus infection. J. Gen. Virol. 71, 767–773 (1990)
Whitney, J. A., Gomez, M., Sheff, D., Kreis, T. E. & Mellman, I. Cytoplasmic coat proteins involved in endosome function. Cell 83, 703–713 (1995)
Aniento, F., Gu, F., Parton, R. G. & Gruenberg, J. An endosomal βCOP is involved in the pH-dependent formation of transport vesicles destined for late endosomes. J. Cell Biol. 133, 29–41 (1996)
Tscherne, D. M., Manicassamy, B. & Garcia-Sastre, A. An enzymatic virus-like particle assay for sensitive detection of virus entry. J. Virol. Methods 10.1016/j.jviromet.2009.10.020 (29 October 2009)
Kutay, U., Bischoff, F. R., Kostka, S., Kraft, R. & Gorlich, D. Export of importin alpha from the nucleus is mediated by a specific nuclear transport factor. Cell 90, 1061–1071 (1997)
Colbran, R. J. Targeting of calcium/calmodulin-dependent protein kinase II. Biochem. J. 378, 1–16 (2004)
Sumi, M. et al. The newly synthesized selective Ca2+/calmodulin dependent protein kinase II inhibitor KN-93 reduces dopamine contents in PC12h cells. Biochem. Biophys. Res. Commun. 181, 968–975 (1991)
Chanda, S. K. et al. Genome-scale functional profiling of the mammalian AP-1 signaling pathway. Proc. Natl Acad. Sci. USA 100, 12153–12158 (2003)
Aza-Blanc, P. et al. Identification of modulators of TRAIL-induced apoptosis via RNAi-based phenotypic screening. Mol. Cell 12, 627–637 (2003)
Sui, B. et al. The use of random homozygous gene perturbation to identify novel host-oriented targets for influenza. Virology 387, 473–481 (2009)
Bader, G. D. & Hogue, C. W. An automated method for finding molecular complexes in large protein interaction networks. BMC Bioinformatics 4, 2 (2003)
Acknowledgements
This work was supported in part by National Institutes of Health (NIH) grants U01 AI1074539, U54 AI057158 (Northeast Biodefense Center), HHSN272200900032C, HHSN266200700010C, 1 PO1 AI058113 and 1R21AI083673. S.S. is supported by a fellowship from the German Research Foundation and D.M.T. is supported by NIH fellowship 1F32AI081428. M.B.O. is supported by NIH training grant 1 T32 AI07647 and Mount Sinai Medical Scientist Training Program T32 GM007280. A.I. is supported by a Japan Society for the Promotion of Science Fellowship. The small molecule screen was performed at the National Screening Laboratory for the Regional Centers of Excellence in Biodefense (NSRB), Harvard Medical School, Boston and was supported by NIH grant U54 AI057159. Confocal laser scanning microscopy was performed at the MSSM-Microscopy Shared Resource Facility, supported with funding from NIH-NCI shared resources grant (5R24 CA095823-04), NSF Major Research Instrumentation grant (DBI-9724504) and NIH shared instrumentation grant (1 S10 RR0 9145-01). We thank R. Fouchier for providing influenza A/Netherlands/602/2009 (H1N1) virus.
Author Contributions R.K. and S.S. are equally contributing first authors; M.L.S. and S.K.C. are equally contributing senior authors. R.K., S.S., A.G.-S., J.A.T.Y., P.P., M.L.S. and S.K.C. designed research; R.K., S.S., A.I., H.-H.H., Su.B., S.E.A., J.G.A., D.M.T., M.B.O., Y.L., Q.G., P.D., L.P. and C.S. performed research; R.K., C.S., P.D., B.P.T., L.M. and G.B. performed the screens; A.O. provided siRNAs; R.K., S.S., B.P.T., M.L.S., S.K.C. and P.D. analysed data; Y.Z., G.B., So.B. and T.I. performed bioinformatics analysis; R.K., S.S., A.G.-S., J.A.T.Y., P.P., M.L.S. and S.K.C. co-wrote the manuscript.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
This file contains Supplementary Methods, Supplementary Data, Supplementary References, Supplementary Figures S1-S14 with Legends and Legends for Supplementary Tables S1-S13. (PDF 14707 kb)
Supplementary Table 1
Scores of 295 confirmed genes required for influenza virus replication (see Supplementary Information file for full Legend). (XLS 211 kb)
Supplementary Table 2
Overrepresented functional processes and protein domains of proteins required for influenza virus replication (see Supplementary Information file for full Legend). (XLS 60 kb)
Supplementary Table 3
Functional classification of biological processes required for influenza virus replication (see Supplementary Information file for full Legend). (XLS 220 kb)
Supplementary Table 4
Overrepresented functional pathways required for influenza virus replication (see Supplementary Information file for full Legend). (XLS 44 kb)
Supplementary Table 5
References for selected functional categories presented in Table 1 (see Supplementary Information file for full Legend). (XLS 30 kb)
Supplementary Table 6
Binary interactions of the host pathogen interaction map for Figure 1C and Supplementary Figure S4 (see Supplementary Information file for full Legend). (XLS 468 kb)
Supplementary Table 7
Overlap between viral host factors required by different RNA viruses (see Supplementary Information file for full Legend). (XLS 37 kb)
Supplementary Table 8
Biochemical complexes that are required by different RNA viruses (see Supplementary Information file for full Legend). (XLS 172 kb)
Supplementary Table 9
Host proteins confirmed to be required for wild-type influenza virus growth and gene expression (see Supplementary Information file for full Legend). (XLS 161 kb)
Supplementary Table 10
Examination of interferon induction in siRNAtransfected cells (see Supplementary Information file for full Legend). (XLS 52 kb)
Supplementary Table 11
Evaluation of host factors that regulate influenza virus entry (see Supplementary Information file for full Legend). (XLS 33 kb)
Supplementary Table 12
Effects of host factor depletion on expression of an influenza virus mini-genome reporter (see Supplementary Information file for full Legend). (XLS 29 kb)
Supplementary Table 13
Expression levels of host factor after siRNA silencing (see Supplementary Information file for full Legend). (XLS 28 kb)
Rights and permissions
About this article
Cite this article
König, R., Stertz, S., Zhou, Y. et al. Human host factors required for influenza virus replication. Nature 463, 813–817 (2010). https://doi.org/10.1038/nature08699
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature08699
This article is cited by
-
UiO-66 nanoparticles combat influenza A virus in mice by activating the RIG-I-like receptor signaling pathway
Journal of Nanobiotechnology (2024)
-
M6PR interacts with the HA2 subunit of influenza A virus to facilitate the fusion of viral and endosomal membranes
Science China Life Sciences (2024)
-
Upregulation of galectin-3 in influenza A virus infection promotes viral RNA synthesis through its association with viral PA protein
Journal of Biomedical Science (2023)
-
An Influenza A virus can evolve to use human ANP32E through altering polymerase dimerization
Nature Communications (2023)
-
Host factor TNK2 is required for influenza virus infection
Genes & Genomics (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.