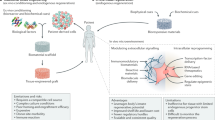
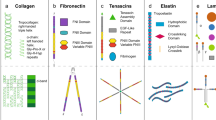
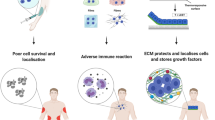
Abstract
Proper tissue function and regeneration rely on robust spatial and temporal control of biophysical and biochemical microenvironmental cues through mechanisms that remain poorly understood. Biomaterials are rapidly being developed to display and deliver stem-cell-regulatory signals in a precise and near-physiological fashion, and serve as powerful artificial microenvironments in which to study and instruct stem-cell fate both in culture and in vivo. Further synergism of cell biological and biomaterials technologies promises to have a profound impact on stem-cell biology and provide insights that will advance stem-cell-based clinical approaches to tissue regeneration.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Blau, H. M., Sacco, A. & Gilbert, P. M. in Essentials of Stem Cell Biology 2nd edn (eds Lanza, R. et al.) 249–257 (Academic, in the press).
Blau, H. M., Sacco, A. & Gilbert, P. M. in Encyclopedia of Stem Cell Research (eds Svendsen, C. & Ebert, A.) (SAGE, in the press).
Daley, G. Q. & Scadden, D. T. Prospects for stem cell-based therapy. Cell 132, 544–548 (2008).
Lutolf, M. P. & Hubbell, J. A. Synthetic biomaterials as instructive extracellular microenvironments for morphogenesis in tissue engineering. Nature Biotechnol. 23, 47–55 (2005).
Scadden, D. T. The stem-cell niche as an entity of action. Nature 441, 1075–1079 (2006).
Morrison, S. J. & Spradling, A. C. Stem cells and niches: mechanisms that promote stem cell maintenance throughout life. Cell 132, 598–611 (2008).
Discher, D. E., Mooney, D. J. & Zandstra, P. W. Growth factors, matrices, and forces combine and control stem cells. Science 324, 1673–1677 (2009).
Guilak, F. et al. Control of stem cell fate by physical interactions with the extracellular matrix. Cell Stem Cell 5, 17–26 (2009).
Chai, C. & Leong, K. W. Biomaterials approach to expand and direct differentiation of stem cells. Mol. Ther. 15, 467–480 (2007).
Saha, K., Pollock, J. F., Schaffer, D. V. & Healy, K. E. Designing synthetic materials to control stem cell phenotype. Curr. Opin. Chem. Biol. 11, 381–387 (2007).
Hwang, N. S., Varghese, S. & Elisseeff, J. Controlled differentiation of stem cells. Adv. Drug Deliv. Rev. 60, 199–214 (2008).
Dawson, E., Mapili, G., Erickson, K., Taqvi, S. & Roy, K. Biomaterials for stem cell differentiation. Adv. Drug Deliv. Rev. 60, 215–228 (2008).
Dellatore, S. M., Garcia, A. S. & Miller, W. M. Mimicking stem cell niches to increase stem cell expansion. Curr. Opin. Biotechnol. 19, 534–540 (2008).
Little, L., Healy, K. E. & Schaffer, D. V. Engineering biomaterials for synthetic neural stem cell microenvironments. Chem. Rev. 108, 1787–1796 (2008).
Burdick, J. A. & Vunjak-Novakovic, G. Engineered microenvironments for controlled stem cell differentiation. Tissue Eng. A 15, 205–219 (2009).
Flaim, C. J., Chien, S. & Bhatia, S. N. An extracellular matrix microarray for probing cellular differentiation. Nature Methods 2, 119–125 (2005).
Soen, Y., Mori, A., Palmer, T. D. & Brown, P. O. Exploring the regulation of human neural precursor cell differentiation using arrays of signaling microenvironments. Mol. Syst. Biol. 2, 37 (2006). This paper presents an approach to probing quantitatively the effects of molecular signals and signal combinations on stem-cell fate decisions.
Derda, R. et al. Defined substrates for human embryonic stem cell growth identified from surface arrays. ACS Chem. Biol. 2, 347–355 (2007).
LaBarge, M. A. et al. Human mammary progenitor cell fate decisions are products of interactions with combinatorial microenvironments. Integr. Biol. 1, 70–79 (2009).
Irvine, D. J., Hue, K. A., Mayes, A. M. & Griffith, L. G. Simulations of cell-surface integrin binding to nanoscale-clustered adhesion ligands. Biophys. J. 82, 120–132 (2002).
Nur-E-Kamal, A. et al. Covalently attached FGF-2 to three-dimensional polyamide nanofibrillar surfaces demonstrates enhanced biological stability and activity. Mol. Cell. Biochem. 309, 157–166 (2008).
Fan, V. H. et al. Tethered epidermal growth factor provides a survival advantage to mesenchymal stem cells. Stem Cells 25, 1241–1251 (2007).
Alberti, K. et al. Functional immobilization of signaling proteins enables control of stem cell fate. Nature Methods 5, 645–650 (2008). This paper demonstrates the relevance of signalling-protein tethering to the fate of (embryonic) stem cells.
Suzuki, T. et al. Highly efficient ex vivo expansion of human hematopoietic stem cells using Delta1-Fc chimeric protein. Stem Cells 24, 2456–2465 (2006).
Beckstead, B. L., Santosa, D. M. & Giachelli, C. M. Mimicking cell–cell interactions at the biomaterial–cell interface for control of stem cell differentiation. J. Biomed. Mater. Res. A 79, 94–103 (2006).
Anderson, D. G., Levenberg, S. & Langer, R. Nanoliter-scale synthesis of arrayed biomaterials and application to human embryonic stem cells. Nature Biotechnol. 22, 863–866 (2004).
Webster, C., Silberstein, L., Hays, A. P. & Blau, H. M. Fast muscle fibers are preferentially affected in Duchenne muscular dystrophy. Cell 52, 503–513 (1988).
Discher, D. E., Janmey, P. & Wang, Y. L. Tissue cells feel and respond to the stiffness of their substrate. Science 310, 1139–1143 (2005).
Paszek, M. J. et al. Tensional homeostasis and the malignant phenotype. Cancer Cell 8, 241–254 (2005).
Engler, A. J., Sen, S., Sweeney, H. L. & Discher, D. E. Matrix elasticity directs stem cell lineage specification. Cell 126, 677–689 (2006). This paper demonstrates the important role of matrix stiffness in the fate of (mesenchymal) stem cells.
Saha, K. et al. Substrate modulus directs neural stem cell behavior. Biophys. J. 95, 4426–4438 (2008).
Boonen, K. J., Rosaria-Chak, K. Y., Baaijens, F. P., van der Schaft, D. W. & Post, M. J. Essential environmental cues from the satellite cell niche: optimizing proliferation and differentiation. Am. J. Physiol. Cell Physiol. 296, C1338−C1345 (2009).
Li, Y. J., Chung, E. H., Rodriguez, R. T., Firpo, M. T. & Healy, K. E. Hydrogels as artificial matrices for human embryonic stem cell self-renewal. J. Biomed. Mater. Res. A 79, 1–5 (2006).
Folkman, J. & Moscona, A. Role of cell shape in growth control. Nature 273, 345–349 (1978).
Chen, C. S., Mrksich, M., Huang, S., Whitesides, G. M. & Ingber, D. E. Geometric control of cell life and death. Science 276, 1425–1428 (1997).
Wozniak, M. A. & Chen, C. S. Mechanotransduction in development: a growing role for contractility. Nature Rev. Mol. Cell Biol. 10, 34–43 (2009).
Chen, C. S., Alonso, J. L., Ostuni, E., Whitesides, G. M. & Ingber, D. E. Cell shape provides global control of focal adhesion assembly. Biochem. Biophys. Res. Commun. 307, 355–361 (2003).
McBeath, R., Pirone, D. M., Nelson, C. M., Bhadriraju, K. & Chen, C. S. Cell shape, cytoskeletal tension, and RhoA regulate stem cell lineage commitment. Dev. Cell 6, 483–495 (2004). This paper highlights the role of cell-shape control in regulating the fate of (mesenchymal) stem cells.
Peerani, R. et al. Niche-mediated control of human embryonic stem cell self-renewal and differentiation. EMBO J. 26, 4744–4755 (2007).
Chin, V. I. et al. Microfabricated platform for studying stem cell fates. Biotechnol. Bioeng. 88, 399–415 (2004).
Mohr, J. C., de Pablo, J. J. & Palecek, S. P. 3-D microwell culture of human embryonic stem cells. Biomaterials 27, 6032–6042 (2006).
Khademhosseini, A. et al. Co-culture of human embryonic stem cells with murine embryonic fibroblasts on microwell-patterned substrates. Biomaterials 27, 5968–5977 (2006).
Karp, J. M. et al. Controlling size, shape and homogeneity of embryoid bodies using poly(ethylene glycol) microwells. Lab Chip 7, 786–794 (2007).
Moeller, H. C., Mian, M. K., Shrivastava, S., Chung, B. G. & Khademhosseini, A. A microwell array system for stem cell culture. Biomaterials 29, 752–763 (2008).
Ungrin, M. D., Joshi, C., Nica, A., Bauwens, C. & Zandstra, P. W. Reproducible, ultra high-throughput formation of multicellular organization from single cell suspension-derived human embryonic stem cell aggregates. PLoS ONE 3, e1565 (2008).
Dykstra, B. et al. High-resolution video monitoring of hematopoietic stem cells cultured in single-cell arrays identifies new features of self-renewal. Proc. Natl Acad. Sci. USA 103, 8185–8190 (2006).
Cordey, M., Limacher, M., Kobel, S., Taylor, V. & Lutolf, M. P. Enhancing the reliability and throughput of neurosphere culture on hydrogel microwell arrays. Stem Cells 26, 2586–2594 (2008).
Lutolf, M. P., Doyonnas, R., Havenstrite, K., Koleckar, K. & Blau, H. M. Perturbation of single hematopoietic stem cell fates in artificial niches. Integr. Biol. 1, 59–69 (2009). This paper presents a combination of in vitro and in vivo methods to deconstruct a stem-cell niche and probe the effects of its individual key components on the fate of single (haematopoietic) stem cells.
Jia, X. & Kiick, K. L. Hybrid multicomponent hydrogels for tissue engineering. Macromol. Biosci. 9, 140–156 (2009).
Albrecht, D. R., Underhill, G. H., Wassermann, T. B., Sah, R. L. & Bhatia, S. N. Probing the role of multicellular organization in three-dimensional microenvironments. Nature Methods 3, 369–375 (2006). This paper presents an interesting approach to the micropatterning of cells in 3D hydrogel microenvironments.
Lutolf, M. P. & Blau, H. M. in Advances in Tissue Engineering (ed. Polak, J.) Ch. 9 (World Scientific, in the press).
Lutolf, M. P. & Blau, H. M. in Mater. Res. Soc. Symp. Proc. Vol. 1140 (eds Prasad Shastri, V., Lendlein, A., Liu, L., Mikos, A. & Mitragotri, S. ) 1140-HH07-07 (Materials Research Society, 2009).
Lutolf, M. P. Artificial ECM: expanding the cell biology toolbox in 3D. Integr. Biol. 1, 235–241 (2009).
Hennink, W. E. & van Nostrum, C. F. Novel crosslinking methods to design hydrogels. Adv. Drug Deliv. Rev. 54, 13–36 (2002).
Kopecek, J. & Yang, J. Y. Hydrogels as smart biomaterials. Polym. Int. 56, 1078–1098 (2007).
Silva, G. A. et al. Selective differentiation of neural progenitor cells by high-epitope density nanofibers. Science 303, 1352–1355 (2004).
Lin, C. C. & Anseth, K. S. PEG hydrogels for the controlled release of biomolecules in regenerative medicine. Pharm. Res. 26, 631–643 (2009).
Underhill, G. H. & Bhatia, S. N. High-throughput analysis of signals regulating stem cell fate and function. Curr. Opin. Chem. Biol. 11, 357–366 (2007).
Gidrol, X. et al. 2D and 3D cell microarrays in pharmacology. Curr. Opin. Pharmacol. 9, 664–668 (2009).
Fernandes, T. G., Diogo, M. M., Clark, D. S., Dordick, J. S. & Cabral, J. M. S. High-throughput cellular microarray platforms: applications in drug discovery, toxicology and stem cell research. Trends Biotechnol. 27, 342–349 (2009).
Lee, M. Y. et al. Three-dimensional cellular microarray for high-throughput toxicology assays. Proc. Natl Acad. Sci. USA 105, 59–63 (2008).
Jongpaiboonkit, L., King, W. J. & Murphy, W. L. Screening for 3D environments that support human mesenchymal stem cell viability using hydrogel arrays. Tissue Eng. A 15, 343–353 (2009).
Sudo, R. et al. Transport-mediated angiogenesis in 3D epithelial coculture. FASEB J. 23, 2155–2164 (2009).
Tam, P. P. L. & Loebel, D. A. F. Gene function in mouse embryogenesis: get set for gastrulation. Nature Rev. Genet. 8, 368–381 (2007).
Whitesides, G. M. The origins and the future of microfluidics. Nature 442, 368–373 (2006).
Chung, B. G. et al. Human neural stem cell growth and differentiation in a gradient-generating microfluidic device. Lab Chip 5, 401–406 (2005).
Choi, N. W. et al. Microfluidic scaffolds for tissue engineering. Nature Mater. 6, 908–915 (2007). This paper is a good example of how microfluidic technology can be used to generate well-controlled protein gradients in 3D cell matrices.
Peret, B. J. & Murphy, W. L. Controllable soluble protein concentration gradients in hydrogel networks. Adv. Funct. Mater. 18, 3410–3417 (2008).
van Noort, D. et al. Stem cells in microfluidics. Biotechnol. Prog. 25, 52–60 (2009).
Gomez-Sjoberg, R., Leyrat, A. A., Pirone, D. M., Chen, C. S. & Quake, S. R. Versatile, fully automated, microfluidic cell culture system. Anal. Chem. 79, 8557–8563 (2007).
Lii, J. et al. Real-time microfluidic system for studying mammalian cells in 3D microenvironments. Anal. Chem. 80, 3640–3647 (2008).
Hahn, M. S., Miller, J. S. & West, J. L. Three-dimensional biochemical and biomechanical patterning of hydrogels for guiding cell behavior. Adv. Mater. 18, 2679–2684 (2006).
Wosnick, J. H. & Shoichet, M. S. Three-dimensional chemical patterning of transparent hydrogels. Chem. Mater. 20, 55–60 (2008).
Kloxin, A. M., Kasko, A. M., Salinas, C. N. & Anseth, K. S. Photodegradable hydrogels for dynamic tuning of physical and chemical properties. Science 324, 59–63 (2009). This paper presents a powerful method of influencing stem-cell fate by locally manipulating the biochemical and biophysical properties of a 3D hydrogel matrix.
Gillette, B. M. et al. In situ collagen assembly for integrating microfabricated three-dimensional cell-seeded matrices. Nature Mater. 7, 636–640 (2008).
Khetani, S. R. & Bhatia, S. N. Engineering tissues for in vitro applications. Curr. Opin. Biotechnol. 17, 524–531 (2006).
Mironov, V., Kasyanov, V., Drake, C. & Markwald, R. R. Organ printing: promises and challenges. Regen. Med. 3, 93–103 (2008).
Lee, W. et al. Three-dimensional bioprinting of rat embryonic neural cells. Neuroreport 20, 798–803 (2009).
Mooney, D. J. & Vandenburgh, H. Cell delivery mechanisms for tissue repair. Cell Stem Cell 2, 205–213 (2008).
Conboy, I. M. et al. Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature 433, 760–764 (2005).
Adams, G. B. et al. Therapeutic targeting of a stem cell niche. Nature Biotechnol. 25, 238–243 (2007).
Zhang, L. et al. Nanoparticles in medicine: therapeutic applications and developments. Clin. Pharmacol. Ther. 83, 761–769 (2008).
Rothenfluh, D. A., Bermudez, H., O'Neil, C. P. & Hubbell, J. A. Biofunctional polymer nanoparticles for intra-articular targeting and retention in cartilage. Nature Mater. 7, 248–254 (2008).
Gu, F. et al. Precise engineering of targeted nanoparticles by using self-assembled biointegrated block copolymers. Proc. Natl Acad. Sci. USA 105, 2586–2591 (2008).
Sacchetti, B. et al. Self-renewing osteoprogenitors in bone marrow sinusoids can organize a hematopoietic microenvironment. Cell 131, 324–336 (2007).
Gomi, K., Kanazashi, M., Lickorish, D., Arai, T. & Davies, J. E. Bone marrow genesis after subcutaneous delivery of rat osteogenic cell-seeded biodegradable scaffolds into nude mice. J. Biomed. Mater. Res. A 71A, 602–607 (2004).
Eilken, H. M., Nishikawa, S.-I. & Schroeder, T. Continuous single-cell imaging of blood generation from haemogenic endothelium. Nature 457, 896–900 (2009).
Glauche, I., Lorenz, R., Hasenclever, D. & Roeder, I. A novel view on stem cell development: analysing the shape of cellular genealogies. Cell Prolif. 42, 248–263 (2009).
Ravin, R. et al. Potency and fate specification in CNS stem cell populations in vitro . Cell Stem Cell 3, 670–680 (2008).
Takahashi, K. & Yamanaka, S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126, 663–676 (2006).
Acknowledgements
We acknowledge a US National Institutes of Health (NIH) postdoctoral training grant (CA09151) (P.M.G.); a Leukemia and Lymphoma Society Fellowship (5367-07) and the European Young Investigator award (M.P.L.); and NIH grants (AG009521, AG020961 and HL096113), a Juvenile Diabetes Research Foundation Grant (34-2008-623), a Muscular Dystrophy Association grant (4320), a California Institute for Regenerative Medicine (CIRM) Tools and Technologies grant (RT1-01001), a Stanford Bio.X award (IIP3-34), and the Baxter Foundation (H.M.B.).
Author information
Authors and Affiliations
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Additional information
Reprints and permissions information is available at http://www.nature.com/reprints.
Correspondence should be addressed to M.P.L. (matthias.lutolf@epfl.ch) or H.M.B. (hblau@stanford.edu).
Rights and permissions
About this article
Cite this article
Lutolf, M., Gilbert, P. & Blau, H. Designing materials to direct stem-cell fate. Nature 462, 433–441 (2009). https://doi.org/10.1038/nature08602
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature08602
This article is cited by
-
Multifunctional hydrogels: advanced therapeutic tools for osteochondral regeneration
Biomaterials Research (2023)
-
Stem cell-based drug delivery strategy for skin regeneration and wound healing: potential clinical applications
Inflammation and Regeneration (2023)
-
Mechanics of the cellular microenvironment as probed by cells in vivo during zebrafish presomitic mesoderm differentiation
Nature Materials (2023)
-
Enzyme-controlled, nutritive hydrogel for mesenchymal stromal cell survival and paracrine functions
Communications Biology (2023)
-
One of Nature’s Basic Laws: Combination-Sharing
Human Arenas (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.