Abstract
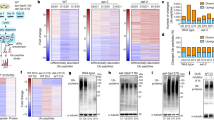
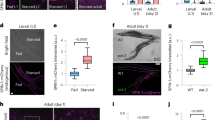
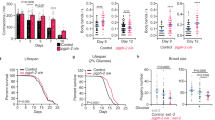
Dietary restriction extends longevity in diverse species, suggesting that there is a conserved mechanism for nutrient regulation and prosurvival responses1. Here we show a role for the HECT (homologous to E6AP carboxy terminus) E3 ubiquitin ligase WWP-1 as a positive regulator of lifespan in Caenorhabditis elegans in response to dietary restriction. We find that overexpression of wwp-1 in worms extends lifespan by up to 20% under conditions of ad libitum feeding. This extension is dependent on the FOXA transcription factor pha-4, and independent of the FOXO transcription factor daf-16. Reduction of wwp-1 completely suppresses the extended longevity of diet-restricted animals. However, the loss of wwp-1 does not affect the long lifespan of animals with compromised mitochondrial function or reduced insulin/IGF-1 signalling. Overexpression of a mutant form of WWP-1 lacking catalytic activity suppresses the increased lifespan of diet-restricted animals, indicating that WWP-1 ubiquitin ligase activity is essential for longevity. Furthermore, we find that the E2 ubiquitin conjugating enzyme, UBC-18, is essential and specific for diet-restriction-induced longevity. UBC-18 interacts with WWP-1 and is required for the ubiquitin ligase activity of WWP-1 and the extended longevity of worms overexpressing wwp-1. Taken together, our results indicate that WWP-1 and UBC-18 function to ubiquitinate substrates that regulate diet-restriction-induced longevity.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Mair, W. & Dillin, A. Aging and survival: the genetics of life span extension by dietary restriction. Annu. Rev. Biochem. 77, 727–754 (2008)
Pirozzi, G. et al. Identification of novel human WW domain-containing proteins by cloning of ligand targets. J. Biol. Chem. 272, 14611–14616 (1997)
Huang, K. et al. A HECT domain ubiquitin ligase closely related to the mammalian protein WWP1 is essential for Caenorhabditis elegans embryogenesis. Gene 252, 137–145 (2000)
Astin, J. W., O’Neil, N. J. & Kuwabara, P. E. Nucleotide excision repair and the degradation of RNA pol II by the Caenorhabditis elegans XPA and Rsp5 orthologues, RAD-3 and WWP-1. DNA Repair (Amst.) 7, 267–280 (2008)
Lakowski, B. & Hekimi, S. The genetics of caloric restriction in Caenorhabditis elegans. Proc. Natl Acad. Sci. USA 95, 13091–13096 (1998)
Mair, W., Panowski, S. H., Shaw, R. J. & Dillin, A. Optimizing dietary restriction for genetic epistasis analysis and gene discovery in C. elegans. PLoS One 4, e4535 (2009)
Bishop, N. A. & Guarente, L. Two neurons mediate diet-restriction-induced longevity in C. elegans. Nature 447, 545–549 (2007)
Panowski, S. H., Wolff, S., Aguilaniu, H., Durieux, J. & Dillin, A. PHA-4/Foxa mediates diet-restriction-induced longevity of C. elegans. Nature 447, 550–555 (2007)
Klass, M. R. Aging in the nematode Caenorhabditis elegans: major biological and environmental factors influencing life span. Mech. Ageing Dev. 6, 413–429 (1977)
Greer, E. L. et al. An AMPK-FOXO pathway mediates longevity induced by a novel method of dietary restriction in C. elegans. Curr. Biol. 17, 1646–1656 (2007)
Lin, K., Hsin, H., Libina, N. & Kenyon, C. Regulation of the Caenorhabditis elegans longevity protein DAF-16 by insulin/IGF-1 and germline signaling. Nature Genet. 28, 139–145 (2001)
Ogg, S. et al. The Fork head transcription factor DAF-16 transduces insulin-like metabolic and longevity signals in C. elegans. Nature 389, 994–999 (1997)
Dillin, A. et al. Rates of behavior and aging specified by mitochondrial function during development. Science 298, 2398–2401 (2002)
Feng, J., Bussiere, F. & Hekimi, S. Mitochondrial electron transport is a key determinant of life span in Caenorhabditis elegans. Dev. Cell 1, 633–644 (2001)
Lee, S. S. et al. A systematic RNAi screen identifies a critical role for mitochondria in C. elegans longevity. Nature Genet. 33, 40–48 (2003)
Kimura, K. D., Tissenbaum, H. A., Liu, Y. & Ruvkun, G. daf-2, an insulin receptor-like gene that regulates longevity and diapause in Caenorhabditis elegans. Science 277, 942–946 (1997)
Bernassola, F., Karin, M., Ciechanover, A. & Melino, G. The HECT family of E3 ubiquitin ligases: multiple players in cancer development. Cancer Cell 14, 10–21 (2008)
Hoppe, T. et al. Activation of a membrane-bound transcription factor by regulated ubiquitin/proteasome-dependent processing. Cell 102, 577–586 (2000)
Qiu, X. & Fay, D. S. ARI-1, an RBR family ubiquitin-ligase, functions with UBC-18 to regulate pharyngeal development in C. elegans. Dev. Biol. 291, 239–252 (2006)
Fay, D. S. et al. The coordinate regulation of pharyngeal development in C. elegans by lin-35/Rb, pha-1, and ubc-18. Dev. Biol. 271, 11–25 (2004)
Fay, D. S., Large, E., Han, M. & Darland, M. lin-35/Rb and ubc-18, an E2 ubiquitin-conjugating enzyme, function redundantly to control pharyngeal morphogenesis in C. elegans. Development 130, 3319–3330 (2003)
Wolkow, C. A., Kimura, K. D., Lee, M. S. & Ruvkun, G. Regulation of C. elegans life-span by insulinlike signaling in the nervous system. Science 290, 147–150 (2000)
Apfeld, J. & Kenyon, C. Regulation of lifespan by sensory perception in Caenorhabditis elegans. Nature 402, 804–809 (1999)
Parkes, T. L. et al. Extension of Drosophila lifespan by overexpression of human SOD1 in motorneurons. Nature Genet. 19, 171–174 (1998)
Alcedo, J. & Kenyon, C. Regulation of C. elegans longevity by specific gustatory and olfactory neurons. Neuron 41, 45–55 (2004)
Libina, N., Berman, J. R. & Kenyon, C. Tissue-specific activities of C. elegans DAF-16 in the regulation of lifespan. Cell 115, 489–502 (2003)
Sheaffer, K. L., Updike, D. L. & Mango, S. E. The Target of Rapamycin pathway antagonizes pha-4/FoxA to control development and aging. Curr. Biol. 18, 1355–1364 (2008)
Brenner, S. The genetics of Caenorhabditis elegans. Genetics 77, 71–94 (1974)
Dillin, A., Crawford, D. K. & Kenyon, C. Timing requirements for insulin/IGF-1 signaling in C. elegans. Science 298, 830–834 (2002)
Hope, I. C. elegans: A Practical Approach Ch. 4 62–63 (Oxford Univ. Press, 1999)
Mello, C. C., Kramer, J. M., Stinchcomb, D. & Ambros, V. Efficient gene transfer in C. elegans: extrachromosomal maintenance and integration of transforming sequences. EMBO J. 10, 3959–3970 (1991)
Leverson, J. D. et al. The APC11 RING-H2 finger mediates E2-dependent ubiquitination. Mol. Biol. Cell 11, 2315–2325 (2000)
Xia, Y., Pao, G. M., Chen, H. W., Verma, I. M. & Hunter, T. Enhancement of BRCA1 E3 ubiquitin ligase activity through direct interaction with the BARD1 protein. J. Biol. Chem. 278, 5255–5263 (2003)
Carrano, A. C., Eytan, E., Hershko, A. & Pagano, M. SKP2 is required for ubiquitin-mediated degradation of the CDK inhibitor p27. Nature Cell Biol. 1, 193–199 (1999)
Kamath, R. S. et al. Systematic functional analysis of the Caenorhabditis elegans genome using RNAi. Nature 421, 231–237 (2003)
Rual, J. F. et al. Toward improving Caenorhabditis elegans phenome mapping with an ORFeome-based RNAi library. Genome Res. 14, 2162–2168 (2004)
Dillin, A., Crawford, D. K. & Kenyon, C. Timing requirements for insulin/IGF-1 signaling in C. elegans. Science 298, 830–834 (2002)
Simmer, F. et al. Genome-wide RNAi of C. elegans using the hypersensitive rrf-3 strain reveals novel gene functions. PLoS Biol. 1, e12 (2003)
Wolff, S. et al. SMK-1, an essential regulator of DAF-16-mediated longevity. Cell 124, 1039–1053 (2006)
Acknowledgements
We thank members of the Dillin laboratory for discussion, and P. Marks and H. Sun for their help. We thank A. Brunet for the solid-plate diet-restriction protocol. wwp-1(ok1102) was generated by the C. elegans Gene Knockout Consortium, and some strains were provided by the Caenorhabditis Genetics Center, which is funded by the National Institutes of Health (NIH) National Center for Research Resources (NCRR). This work was supported by grants from the National Cancer Institute (CA 14195, CA 54418 and CA 82683) to T.H., and grants from the National Institute of Diabetes and Digestive and Kidney Diseases (DK 070696) and the National Institute on Aging (AG 027463 and AG 032560), and the Ellison Medical and Glenn Medical Foundations to A.D. A.C.C. was supported by an American Cancer Society Postdoctoral Fellowship and The Rossi Endowment. T.H. is a Frank and Else Schilling American Cancer Society Professor.
Author Contributions A.C.C. designed the experiments and analysed the data. A.C.C. and Z.L. performed the experiments. A.D. and T.H. supervised the design and data interpretation. The manuscript was written by A.C.C. and edited by A.D. and T.H. All authors discussed the results and commented on the manuscript.
Author information
Authors and Affiliations
Corresponding author
Supplementary information
Supplementary information
This file contains Supplementary Tables 1-7, Supplementary Figures 1-15 with Legends and Supplementary References. (PDF 12213 kb)
Rights and permissions
About this article
Cite this article
Carrano, A., Liu, Z., Dillin, A. et al. A conserved ubiquitination pathway determines longevity in response to diet restriction. Nature 460, 396–399 (2009). https://doi.org/10.1038/nature08130
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature08130
This article is cited by
-
Identification of healthspan-promoting genes in Caenorhabditis elegans based on a human GWAS study
Biogerontology (2022)
-
“High-Throughput Characterization of Region-Specific Mitochondrial Function and Morphology”
Scientific Reports (2017)
-
RLIP76 Inhibition: A Promising Developmental Therapy for Neuroblastoma
Pharmaceutical Research (2017)
-
Ubiquitin sets the timer: impacts on aging and longevity
Nature Structural & Molecular Biology (2014)
-
A Krüppel-like factor downstream of the E3 ligase WWP-1 mediates dietary-restriction-induced longevity in Caenorhabditis elegans
Nature Communications (2014)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.