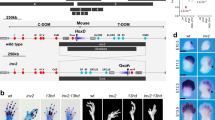
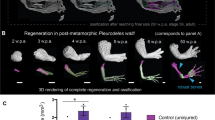
Abstract
Phocomelia is a devastating, rare congenital limb malformation in which the long bones are shorter than normal, with the upper portion of the limb being most severely affected. In extreme cases, the hands or fingers are attached directly to the shoulder and the most proximal elements (those closest to the shoulder) are entirely missing. This disorder, previously known in both autosomal recessive and sporadic forms, showed a marked increase in incidence in the early 1960s due to the tragic toxicological effects of the drug thalidomide, which had been prescribed as a mild sedative1,2. This human birth defect is mimicked in developing chick limb buds exposed to X-irradiation3,4,5. Both X-irradiation5 and thalidomide-induced phocomelia5,6 have been interpreted as patterning defects in the context of the progress zone model, which states that a cell’s proximodistal identity is determined by the length of time spent in a distal limb region termed the ‘progress zone’7. Indeed, studies of X-irradiation-induced phocomelia have served as one of the two major experimental lines of evidence supporting the validity of the progress zone model. Here, using a combination of molecular analysis and lineage tracing in chick, we show that X-irradiation-induced phocomelia is fundamentally not a patterning defect, but rather results from a time-dependent loss of skeletal progenitors. Because skeletal condensation proceeds from the shoulder to fingers (in a proximal to distal direction), the proximal elements are differentially affected in limb buds exposed to radiation at early stages. This conclusion changes the framework for considering the effect of thalidomide and other forms of phocomelia, suggesting the possibility that the aetiology lies not in a defect in the patterning process, but rather in progenitor cell survival and differentiation. Moreover, molecular evidence that proximodistal patterning is unaffected after X-irradiation does not support the predictions of the progress zone model.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Lenz, W. Thalidomide and congenital abnormalities. Lancet 279, 45–46 (1962)
Toms, D. Thalidomide and congenital limb abnormalities. Lancet 280, 400–401 (1962)
Goff, R. A. The relation of development status of limb formation of X-radiation sensitivity in chick embryos. I. Groso study. J. Exp. Zool. 151, 177–200 (1962)
Pinot, M. Developpement de l’ebauche des Membres apres traitement a l’yperite azotee, irradiation aux rayons X et culture in vitro. Etude comparative chez l’embryon de poulet. Annal. Embryol. Morphog. 3, 215–234 (1970)
Wolpert, L., Tickle, C. & Sampford, M. The effect of cell killing by X-irradiation on pattern formation in the chick limb. J. Embryol. Exp. Morphol. 50, 175–193 (1979)
Tabin, C. J. A developmental model for thalidomide defects. Nature 396, 322–323 (1998)
Summerbell, D., Lewis, J. H. & Wolpert, L. Positional information in chick limb morphogenesis. Nature 244, 492–496 (1973)
Niswander, L., Tickle, C., Vogel, A., Booth, I. & Martin, G. R. FGF-4 replaces the apical ectodermal ridge and directs outgrowth and patterning of the limb. Cell 75, 579–587 (1993)
Niswander, L., Jeffrey, S., Martin, G. R. & Tickle, C. A positive feedback loop coordinates growth and patterning in the vertebrate limb. Nature 371, 609–612 (1994)
Laufer, E., Nelson, C. E., Johnson, R. L., Morgan, B. A. & Tabin, C. Sonic hedgehog and Fgf-4 act through a signaling cascade and feedback loop to integrate growth and patterning of the developing limb bud. Cell 79, 993–1003 (1994)
Shen, H. et al. Chicken transcription factor AP-2: cloning, expression and its role in outgrowth of facial prominences and limb buds. Dev. Biol. 188, 248–266 (1997)
Tabin, C. & Wolpert, L. Rethinking the proximodistal axis of the vertebrate limb in the molecular era. Genes Dev. 21, 1433–1442 (2007)
Nelson, C. E. et al. Analysis of Hox gene expression in the chick limb bud. Development 122, 1449–1466 (1996)
Choudhury, A., Cuddihy, A. & Bristow, R. G. Radiation and new molecular agents part I: targeting ATM-ATR checkpoints, DNA repair, and the proteasome. Semin. Radiat. Oncol. 16, 51–58 (2006)
Akiyama, H., Chaboissier, M. C., Martin, J. F., Schedl, A. & de Crombrugghe, B. The transcription factor Sox9 has essential roles in successive steps of the chondrocyte differentiation pathway and is required for expression of Sox5 and Sox6. Genes Dev. 16, 2813–2828 (2002)
Sun, X., Mariani, F. V. & Martin, G. R. Functions of FGF signalling from the apical ectodermal ridge in limb development. Nature 418, 501–508 (2002)
Mariani, F. V. & Martin, G. R. Deciphering skeletal patterning: clues from the limb. Nature 423, 319–325 (2003)
Biggers, J. D. & Gwatkin, R. B. Effect of X-rays on the morphogenesis of the embryonic chick tibiotarsus. Nature 202, 152–154 (1964)
Sato, K., Koizumi, Y., Takahashi, M., Kuroiwa, A. & Tamura, K. Specification of cell fate along the proximal–distal axis in the developing chick limb bud. Development 134, 1397–1406 (2007)
Dudley, A. T., Ros, M. A. & Tabin, C. J. A re-examination of proximodistal patterning during vertebrate limb development. Nature 418, 539–544 (2002)
Mercader, N. et al. Opposing RA and FGF signals control proximodistal vertebrate limb development through regulation of Meis genes. Development 127, 3961–3970 (2000)
Knobloch, J., Shaughnessy, J. D. & Ruther, U. Thalidomide induces limb deformities by perturbing the Bmp/Dkk1/Wnt signaling pathway. FASEB J. 21, 1410–1421 (2007)
Knobloch, J., Schmitz, I., Gotz, K., Schulze-Osthoff, K. & Ruther, U. Thalidomide induces limb anomalies by PTEN stabilization, Akt suppression, and stimulation of caspase-dependent cell death. Mol. Cell. Biol. 28, 529–538 (2008)
Therapontos, C., Erskine, L., Gardner, E., Figg, W. & Vargesson, N. Thalidomide induces limb defects by preventing angiogenic outgrowth during early limb formation. Proc. Natl Acad. Sci. USA. 106, 8573–8578 (2009)
Salzgeber, B. Comparative study of the effects of nitrogen mustard on mesodermal and ectodermal limb bud components of chick embryos. J. Embryol. Exp. Morphol. 22, 373–394 (1969)
Salzgeber, B. Ectodermal-mesodermal interactions in chick embryo limb buds treated with nitrogen mustard. Dev. Growth Differ. 17, 295–296 (1975)
Wilkinson, D. G. & Nieto, M. A. Detection of messenger RNA by in situ hybridization to tissue sections and whole mounts. Methods Enzymol. 225, 361–373 (1993)
McLeod, M. J. Differential staining of cartilage and bone in whole mouse fetuses by alcian blue and alizarin red S. Teratology 22, 299–301 (1980)
Riddle, R. D., Johnson, R. L., Laufer, E. & Tabin, C. Sonic hedgehog mediates the polarizing activity of the ZPA. Cell 75, 1401–1416 (1993)
Healy, C., Uwanogho, D. & Sharpe, P. T. Regulation and role of Sox9 in cartilage formation. Dev. Dyn. 215, 69–78 (1999)
Acknowledgements
We would like to thank J. G. Arozamena and D. Connor for help with the irradiation machine and members of the Tabin-Cepko and Ros laboratories for discussions and ideas. Work in the laboratory of C.J.T. is supported by a grant, R37 HD032443, from the NIH. J.L.G. is supported by grant F32HD057701 from the National Institute of Child Health and Human Development. I.D. and M.A.R. are supported by BFU2008-00397 from the Spanish Ministry of Science and Innovation.
Author Contributions J.L.G. performed the shielding experiments. I.D. performed the grafting experiments. J.L.G., I.D., C.J.T. and M.A.R. came up with ideas, analysed data and wrote the paper. C.J.T. and M.A.R. oversaw the research.
Author information
Authors and Affiliations
Corresponding author
Supplementary information
Supplementary Figures
This file contains Supplementary Figures 1-6 with Legends. (PDF 6671 kb)
Rights and permissions
About this article
Cite this article
Galloway, J., Delgado, I., Ros, M. et al. A reevaluation of X-irradiation-induced phocomelia and proximodistal limb patterning. Nature 460, 400–404 (2009). https://doi.org/10.1038/nature08117
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature08117
This article is cited by
-
Sonic hedgehog-expressing cells in the developing limb measure time by an intrinsic cell cycle clock
Nature Communications (2014)
-
Vertebrate limb bud development: moving towards integrative analysis of organogenesis
Nature Reviews Genetics (2009)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.