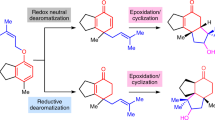
Abstract
From menthol to cholesterol to Taxol, terpenes are a ubiquitous group of molecules (over 55,000 members isolated so far) that have long provided humans with flavours, fragrances, hormones, medicines and even commercial products such as rubber1. Although they possess a seemingly endless variety of architectural complexities, the biosynthesis of terpenes often occurs in a unified fashion as a ‘two-phase’ process2,3. In the first phase (the cyclase phase), simple linear hydrocarbon phosphate building blocks are stitched together by means of ‘prenyl coupling’, followed by enzymatically controlled molecular cyclizations and rearrangements. In the second phase (the oxidase phase), oxidation of alkenes and carbon–hydrogen bonds results in a large array of structural diversity. Although organic chemists have made great progress in developing the logic3,4,5 needed for the cyclase phase of terpene synthesis, particularly in the area of polyene cyclizations6, much remains to be learned if the oxidase phase is to be mimicked in the laboratory. Here we show how the logic of terpene biosynthesis has inspired the highly efficient and stereocontrolled syntheses of five oxidized members of the eudesmane family of terpenes in a modicum of steps by a series of simple carbocycle-forming reactions followed by multiple site-selective inter- and intramolecular carbon–hydrogen oxidations. This work establishes an intellectual framework in which to conceive the laboratory synthesis of other complex terpenes using a ‘two-phase’ approach.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Nicolaou, K. C. & Montagnon, T. Molecules That Changed the World (Wiley-VCH, 2008)
Davis, E. M. & Croteau, R. Cyclization enzymes in the biosynthesis of monoterpenes, sesquiterpenes and diterpenes. Top. Curr. Chem. 209, 53–95 (2000)
Maimone, T. J. & Baran, P. S. Modern synthetic efforts toward biologically active terpenes. Nature Chem. Biol. 3, 396–407 (2007)
Corey, E. J. & Cheng, X. M. The Logic of Chemical Synthesis (Wiley, 1995)
Wilson, R. & Danishefsky, S. J. Pattern recognition in retrosynthetic analysis: snapshots in total synthesis. J. Org. Chem. 72, 4293–4305 (2007)
Yoder, R. A. & Johnston, J. N. A case study in biomimetic total synthesis: polyolefin carbocyclizations to terpenes and steroids. Chem. Rev. 105, 4730–4756 (2005)
Ruzicka, L. Isoprene rule and the biogenesis of terpenic compounds. Experentia 9, 357–367 (1953)
Eschenmoser, A. & Arigoni, D. Revisited after 50 years: the ‘Stereochemical interpretation of the biogenetic isoprene rule for the triterpenes’. Helv. Chim. Acta 88, 3011–3050 (2005)
Jennewein, S., Rithner, C. D., Williams, R. M. & Croteau, R. B. Taxol biosynthesis: taxane 13α-hydroxylase is a cytochrome P450-dependent monooxygenase. Proc. Natl Acad. Sci. USA 98, 13595–13600 (2001)
Denis, J. N. et al. Highly efficient, practical approach to natural Taxol. J. Am. Chem. Soc. 110, 5917–5919 (1988)
Mello, R., Fiorentino, M., Fusco, C. & Curci, R. Oxidations by methyl(trifluoromethyl)dioxirane. 2. Oxyfunctionalization of saturated hydrocarbons. J. Am. Chem. Soc. 111, 6749–6757 (1989)
Costas, M., Mehn, M. P., Jensen, M. P. & Que, L. Dioxygen activation at mononuclear nonheme iron active sites: enzymes, models, and intermediates. Chem. Rev. 104, 939–986 (2004)
Groves, J. T., Bonchio, M., Carofiglio, T. & Shalyaev, K. Rapid catalytic oxygenation of hydrocarbons by ruthenium pentafluorophenylporphyrin complexes: evidence for the involvement of a Ru(III) intermediate. J. Am. Chem. Soc. 118, 8961–8962 (1996)
Wender, P. A., Hilinski, M. K. & Mayweg, A. V. W. Late-stage intermolecular C-H activation for lead diversification: a highly chemoselective oxyfunctionalization of the C-9 position of potent bryostatin analogues. Org. Lett. 7, 79–82 (2005)
Brodsky, B. H. & Du Bois, J. Oxaziridine-mediated catalytic hydroxylation of unactivated 3° C–H bonds using hydrogen peroxide. J. Am. Chem. Soc. 127, 15391–15393 (2005)
Yang, J., Gabriele, B., Belvedere, S., Huang, Y. & Breslow, R. Catalytic oxidations of steroid substrates by artificial cytochrome P-450 enzymes. J. Org. Chem. 67, 5057–5067 (2002)
Chen, M. S. & White, M. C. A predictably selective aliphatic C-H oxidation reaction for complex molecule synthesis. Science 318, 783–787 (2007)
Wu, Q.-X., Shi, Y.-P. & Jia, Z.-J. Eudesmane sesquiterpenoids from the Asteraceae family. Nat. Prod. Rep. 23, 699–734 (2006)
Cardona, L., Garcia, B., Gimenez, E. & Pedro, J. R. A shorter route to the synthesis of (+)-junenol, isojunenol, and their coumarate esters from (-)-santonin. Tetrahedron 48, 851–860 (1992)
Levine, S. R., Krout, M. R. & Stoltz, B. M. Catalytic enantioselective approach to the eudesmane sesquiterpenoids: total synthesis of (+)-carissone. Org. Lett. 11, 289–292 (2009)
Paknikar, S. K., Dhekne, V. V. & Joshi, G. D. Synthesis of 4α, 6α-dihydroxyeudesmane: revision of stereochemistry at C-4 of ajanol. Indian J. Chem. 15B, 86–87 (1977)
Zhao, P.-J., Li, G.-H. & Shen, Y.-M. New chemical constituents from the endophyte Streptomyces species LR4612 cultivated on Maytenus hookeri. Chem. Biodivers. 3, 337–342 (2006)
Irwin, M. A. & Geissman, T. A. Sesquiterpene alcohols from Artemisia pygmaea . Phytochemistry 12, 849–852 (1973)
De Marino, S. et al. New sesquiterpene lactones from Laurus nobilis leaves as inhibitors of nitric oxide production. Planta Med. 71, 706–710 (2005)
Betancort, J. M. & Barbas, C. F. Catalytic direct asymmetric Michael reactions: taming naked aldehyde donors. Org. Lett. 3, 3737–3740 (2001)
Chi, Y. & Gellman, S. H. Diphenylprolinol methyl ether: a highly enantioselective catalyst for Michael addition of aldehydes to simple enones. Org. Lett. 7, 4253–4256 (2005)
Chen, K., Richter, J. M. & Baran, P. S. 1,3-diol synthesis via controlled, radical-mediated C-H functionalization. J. Am. Chem. Soc. 130, 7247–7249 (2008)
Schreiber, J. & Eschenmoser, A. Über die relative Geschwindigkeit der Chromosäureoxydation sekundärer, alicyclischer Alkohole. Vorläufige Mitteilung. Helv. Chim. Acta 38, 1529–1536 (1955)
Fraunhoffer, K. J., Bachovchin, D. A. & White, M. C. Hydrocarbon oxidation vs C-C bond-forming approaches for efficient syntheses of oxygenated molecules. Org. Lett. 7, 223–226 (2005)
Davies, H. M. L. & Manning, J. R. Catalytic C–H functionalization by metal carbenoid and nitrenoid insertion. Nature 451, 417–424 (2008)
Acknowledgements
We are grateful to A. Eschenmoser for discussions and to Bristol-Myers Squibb for financial support. M. Morón Galán and Y. Ishihara are acknowledged for technical contributions to the early stages of this project. We are grateful to B. Shi and A. Reingold for assistance with high-performance liquid chromatography and X-ray crystallographic analyses, respectively.
Author information
Authors and Affiliations
Corresponding author
Additional information
The X-ray crystallographic coordinates for the structures reported in this paper have been deposited at the Cambridge Crystallographic Data Centre, under deposition numbers CCDC 718278 (5), CCDC 719000 (6), CCDC 718279 (15), CCDC 718280 (19), CCDC 718281 (21) and CCDC 718282 (22). These data can be obtained free of charge from the Cambridge Crystallographic Data Centre (http://www.ccdc.cam.ac.uk/data_request/cif).
Supplementary information
Supplementary Information
This file contains a Supplementary Discussion, Supplementary Methods, Supplementary Figures S1-S5, Supplementary References and Supplementary Data. (PDF 4149 kb)
Rights and permissions
About this article
Cite this article
Chen, K., Baran, P. Total synthesis of eudesmane terpenes by site-selective C–H oxidations. Nature 459, 824–828 (2009). https://doi.org/10.1038/nature08043
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature08043
This article is cited by
-
Strategic application of C–H oxidation in natural product total synthesis
Nature Reviews Chemistry (2023)
-
Unified short syntheses of oxygenated tricyclic aromatic diterpenes by radical cyclization with a photoredox catalyst
Communications Chemistry (2023)
-
The interplay of polar effects in controlling the selectivity of radical reactions
Nature Synthesis (2022)
-
Total synthesis of nine longiborneol sesquiterpenoids using a functionalized camphor strategy
Nature Chemistry (2022)
-
Total synthesis of terpenes via palladium-catalysed cyclization strategy
Nature Chemistry (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.