Abstract
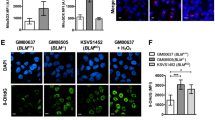
Mice deficient in the Polycomb repressor Bmi1 develop numerous abnormalities including a severe defect in stem cell self-renewal, alterations in thymocyte maturation and a shortened lifespan. Previous work has implicated de-repression of the Ink4a/Arf (also known as Cdkn2a) locus as mediating many of the aspects of the Bmi1-/- phenotype. Here we demonstrate that cells derived from Bmi1-/- mice also have impaired mitochondrial function, a marked increase in the intracellular levels of reactive oxygen species and subsequent engagement of the DNA damage response pathway. Furthermore, many of the deficiencies normally observed in Bmi1-/- mice improve after either pharmacological treatment with the antioxidant N-acetylcysteine or genetic disruption of the DNA damage response pathway by Chk2 (also known as Chek2) deletion. These results demonstrate that Bmi1 has an unexpected role in maintaining mitochondrial function and redox homeostasis and indicate that the Polycomb family of proteins can coordinately regulate cellular metabolism with stem and progenitor cell function.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Park, I. K. et al. Bmi-1 is required for maintenance of adult self-renewing haematopoietic stem cells. Nature 423, 302–305 (2003)
Molofsky, A. V. et al. Bmi-1 dependence distinguishes neural stem cell self-renewal from progenitor proliferation. Nature 425, 962–967 (2003)
van der Lugt, N. M. et al. Posterior transformation, neurological abnormalities, and severe hematopoietic defects in mice with a targeted deletion of the bmi-1 proto-oncogene. Genes Dev. 8, 757–769 (1994)
Jacobs, J. J., Kieboom, K., Marino, S., DePinho, R. A. & van Lohuizen, M. The oncogene and Polycomb-group gene bmi-1 regulates cell proliferation and senescence through the ink4a locus. Nature 397, 164–168 (1999)
Bruggeman, S. W. et al. Ink4a and Arf differentially affect cell proliferation and neural stem cell self-renewal in Bmi1-deficient mice. Genes Dev. 19, 1438–1443 (2005)
Molofsky, A. V., He, S., Bydon, M., Morrison, S. J. & Pardal, R. Bmi-1 promotes neural stem cell self-renewal and neural development but not mouse growth and survival by repressing the p16Ink4a and p19Arf senescence pathways. Genes Dev. 19, 1432–1437 (2005)
Bracken, A. P. et al. The Polycomb group proteins bind throughout the INK4A-ARF locus and are disassociated in senescent cells. Genes Dev. 21, 525–530 (2007)
Oguro, H. et al. Differential impact of Ink4a and Arf on hematopoietic stem cells and their bone marrow microenvironment in Bmi1-deficient mice. J. Exp. Med. 203, 2247–2253 (2006)
Tothova, Z. et al. FoxOs are critical mediators of hematopoietic stem cell resistance to physiologic oxidative stress. Cell 128, 325–339 (2007)
Ito, K. et al. Regulation of oxidative stress by ATM is required for self-renewal of haematopoietic stem cells. Nature 431, 997–1002 (2004)
Kiel, M. J. et al. SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell 121, 1109–1121 (2005)
Bracken, A. P., Dietrich, N., Pasini, D., Hansen, K. H. & Helin, K. Genome-wide mapping of Polycomb target genes unravels their roles in cell fate transitions. Genes Dev. 20, 1123–1136 (2006)
Fasano, C. A. et al. shRNA knockdown of Bmi-1 reveals a critical role for p21-Rb pathway in NSC self-renewal during development. Cell Stem Cell 1, 87–99 (2007)
Balaban, R. S., Nemoto, S. & Finkel, T. Mitochondria, oxidants, and aging. Cell 120, 483–495 (2005)
Takahashi, A. et al. Mitogenic signalling and the p16INK4a-Rb pathway cooperate to enforce irreversible cellular senescence. Nature Cell Biol. 8, 1291–1297 (2006)
Lombard, D. B. et al. DNA repair, genome stability, and aging. Cell 120, 497–512 (2005)
Adams, M. M. & Carpenter, P. B. Tying the loose ends together in DNA double strand break repair with 53BP1. Cell Div 1, 19 (2006)
Takai, H. et al. Chk2-deficient mice exhibit radioresistance and defective p53-mediated transcription. EMBO J. 21, 5195–5205 (2002)
Hirao, A. et al. Chk2 is a tumor suppressor that regulates apoptosis in both an ataxia telangiectasia mutated (ATM)-dependent and an ATM-independent manner. Mol. Cell. Biol. 22, 6521–6532 (2002)
Okada, H. et al. Survivin loss in thymocytes triggers p53-mediated growth arrest and p53-independent cell death. J. Exp. Med. 199, 399–410 (2004)
Zaugg, K. et al. Cross-talk between Chk1 and Chk2 in double-mutant thymocytes. Proc. Natl Acad. Sci. USA 104, 3805–3810 (2007)
Rossi, D. J. et al. Deficiencies in DNA damage repair limit the function of haematopoietic stem cells with age. Nature 447, 725–729 (2007)
Reese, J. S., Liu, L. & Gerson, S. L. Repopulating defect of mismatch repair-deficient hematopoietic stem cells. Blood 102, 1626–1633 (2003)
Nijnik, A. et al. DNA repair is limiting for haematopoietic stem cells during ageing. Nature 447, 686–690 (2007)
Viale, A. et al. Cell-cycle restriction limits DNA damage and maintains self-renewal of leukaemia stem cells. Nature 457, 51–56 (2009)
Rossi, D. J., Jamieson, C. H. & Weissman, I. L. Stems cells and the pathways to aging and cancer. Cell 132, 681–696 (2008)
Janzen, V. et al. Stem-cell ageing modified by the cyclin-dependent kinase inhibitor p16INK4a. Nature 443, 421–426 (2006)
Molofsky, A. V. et al. Increasing p16INK4a expression decreases forebrain progenitors and neurogenesis during ageing. Nature 443, 448–452 (2006)
Krishnamurthy, J. et al. p16INK4a induces an age-dependent decline in islet regenerative potential. Nature 443, 453–457 (2006)
Collado, M., Blasco, M. A. & Serrano, M. Cellular senescence in cancer and aging. Cell 130, 223–233 (2007)
Finkel, T., Serrano, M. & Blasco, M. A. The common biology of cancer and ageing. Nature 448, 767–774 (2007)
Ferrick, D. A., Neilson, A. & Beeson, C. Advances in measuring cellular bioenergetics using extracellular flux. Drug Discov. Today 13, 268–274 (2008)
Struthers, L., Patel, R., Clark, J. & Thomas, S. Direct detection of 8-oxodeoxyguanosine and 8-oxoguanine by avidin and its analogues. Anal. Biochem. 255, 20–31 (1998)
Neumann, C. A. et al. Essential role for the peroxiredoxin Prdx1 in erythrocyte antioxidant defence and tumour suppression. Nature 424, 561–565 (2003)
Sharpless, N. E. et al. Loss of p16Ink4a with retention of p19Arf predisposes mice to tumorigenesis. Nature 413, 86–91 (2001)
Smith, A. L., Ellison, F. M., McCoy, J. P. & Chen, J. c-Kit expression and stem cell factor-induced hematopoietic cell proliferation are up-regulated in aged B6D2F1 mice. J. Gerontol. A Biol. Sci. Med. Sci. 60, 448–456 (2005)
Schieke, S. M. et al. The mammalian target of rapamycin (mTOR) pathway regulates mitochondrial oxygen consumption and oxidative capacity. J. Biol. Chem. 281, 27643–27652 (2006)
TeKippe, M., Harrison, D. E. & Chen, J. Expansion of hematopoietic stem cell phenotype and activity in Trp53-null mice. Exp. Hematol. 31, 521–527 (2003)
Acknowledgements
We are grateful to M. Clarke for providing the initial supply of Bmi1-/- mice, to J. Moss for the gift of anti-PARP antibodies and to M. Daniels and the NHLBI electron microscope core for their assistance. This work was supported by funding from the NIH intramural program and the Ellison Medical Foundation.
Author information
Authors and Affiliations
Corresponding authors
Supplementary information
Supplementary Information
This file contains Supplementary Figures 1-13 with Legends, Supplementary Methods and Materials and Supplementary References. (PDF 1043 kb)
Rights and permissions
About this article
Cite this article
Liu, J., Cao, L., Chen, J. et al. Bmi1 regulates mitochondrial function and the DNA damage response pathway. Nature 459, 387–392 (2009). https://doi.org/10.1038/nature08040
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature08040
This article is cited by
-
Emerging roles of mitochondrial functions and epigenetic changes in the modulation of stem cell fate
Cellular and Molecular Life Sciences (2024)
-
Trichodysplasia spinulosa polyomavirus small T antigen synergistically modulates S6 protein translation and DNA damage response pathways to shape host cell environment
Virus Genes (2022)
-
Hippo/YAP signaling pathway protects against neomycin-induced hair cell damage in the mouse cochlea
Cellular and Molecular Life Sciences (2022)
-
The plasminogen receptor directs maintenance of spermatogonial stem cells by targeting BMI1
Molecular Biology Reports (2022)
-
Bmi-1 alleviates adventitial fibroblast senescence by eliminating ROS in pulmonary hypertension
BMC Pulmonary Medicine (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.