Abstract
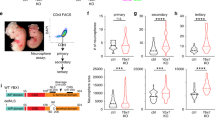
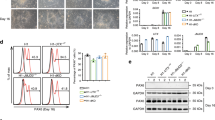
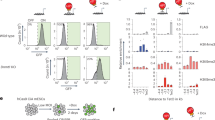
Epigenetic mechanisms that maintain neurogenesis throughout adult life remain poorly understood1. Trithorax group (trxG) and Polycomb group (PcG) gene products are part of an evolutionarily conserved chromatin remodelling system that activate or silence gene expression, respectively2. Although PcG member Bmi1 has been shown to be required for postnatal neural stem cell self-renewal3,4, the role of trxG genes remains unknown. Here we show that the trxG member Mll1 (mixed-lineage leukaemia 1) is required for neurogenesis in the mouse postnatal brain. Mll1-deficient subventricular zone neural stem cells survive, proliferate and efficiently differentiate into glial lineages; however, neuronal differentiation is severely impaired. In Mll1-deficient cells, early proneural Mash1 (also known as Ascl1) and gliogenic Olig2 expression are preserved, but Dlx2, a key downstream regulator of subventricular zone neurogenesis, is not expressed. Overexpression of Dlx2 can rescue neurogenesis in Mll1-deficient cells. Chromatin immunoprecipitation demonstrates that Dlx2 is a direct target of MLL in subventricular zone cells. In differentiating wild-type subventricular zone cells, Mash1, Olig2 and Dlx2 loci have high levels of histone 3 trimethylated at lysine 4 (H3K4me3), consistent with their transcription. In contrast, in Mll1-deficient subventricular zone cells, chromatin at Dlx2 is bivalently marked by both H3K4me3 and histone 3 trimethylated at lysine 27 (H3K27me3), and the Dlx2 gene fails to properly activate. These data support a model in which Mll1 is required to resolve key silenced bivalent loci in postnatal neural precursors to the actively transcribed state for the induction of neurogenesis, but not for gliogenesis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Hsieh, J. & Gage, F. H. Epigenetic control of neural stem cell fate. Curr. Opin. Genet. Dev. 14, 461–469 (2004)
Schuettengruber, B., Chourrout, D., Vervoort, M., Leblanc, B. & Cavalli, G. Genome regulation by polycomb and trithorax proteins. Cell 128, 735–745 (2007)
Molofsky, A. V. et al. Bmi-1 dependence distinguishes neural stem cell self-renewal from progenitor proliferation. Nature 425, 962–967 (2003)
Fasano, C. A. et al. shRNA knockdown of Bmi-1 reveals a critical role for p21-Rb pathway in NSC self-renewal during development. Cell Stem Cell 1, 87–99 (2007)
Lim, D. A. et al. In vivo transcriptional profile analysis reveals RNA splicing and chromatin remodeling as prominent processes for adult neurogenesis. Mol. Cell. Neurosci. 31, 131–148 (2006)
Daser, A. & Rabbitts, T. H. Extending the repertoire of the mixed-lineage leukemia gene MLL in leukemogenesis. Genes Dev. 18, 965–974 (2004)
Yu, B. D., Hess, J. L., Horning, S. E., Brown, G. A. & Korsmeyer, S. J. Altered Hox expression and segmental identity in Mll-mutant mice. Nature 378, 505–508 (1995)
Jude, C. D. et al. Unique and independent roles for MLL in adult hematopoietic stem cells and progenitors. Cell Stem Cell 1, 324–337 (2007)
Zhuo, L. et al. hGFAP-cre transgenic mice for manipulation of glial and neuronal function in vivo . Genesis 31, 85–94 (2001)
Han, Y. G. et al. Hedgehog signaling and primary cilia are required for the formation of adult neural stem cells. Nature Neurosci. 11, 277–284 (2008)
Luskin, M. B. Restricted proliferation and migration of postnatally generated neurons derived from the forebrain subventricular zone. Neuron 11, 173–189 (1993)
Lois, C. & Alvarez-Buylla, A. Long-distance neuronal migration in the adult mammalian brain. Science 264, 1145–1148 (1994)
Doetsch, F., Caille, I., Lim, D. A., Garcia-Verdugo, J. M. & Alvarez-Buylla, A. Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell 97, 703–716 (1999)
Wichterle, H., Garcia-Verdugo, J. M. & Alvarez-Buylla, A. Direct evidence for homotypic, glia-independent neuronal migration. Neuron 18, 779–791 (1997)
Marshall, C. A., Suzuki, S. O. & Goldman, J. E. Gliogenic and neurogenic progenitors of the subventricular zone: who are they, where did they come from, and where are they going? Glia 43, 52–61 (2003)
Picard-Riera, N. et al. Experimental autoimmune encephalomyelitis mobilizes neural progenitors from the subventricular zone to undergo oligodendrogenesis in adult mice. Proc. Natl Acad. Sci. USA 99, 13211–13216 (2002)
Menn, B. et al. Origin of oligodendrocytes in the subventricular zone of the adult brain. J. Neurosci. 26, 7907–7918 (2006)
Spassky, N. et al. Adult ependymal cells are postmitotic and are derived from radial glial cells during embryogenesis. J. Neurosci. 25, 10–18 (2005)
Lu, Q. R. et al. Common developmental requirement for Olig function indicates a motor neuron/oligodendrocyte connection. Cell 109, 75–86 (2002)
Zhou, Q. & Anderson, D. J. The bHLH transcription factors OLIG2 and OLIG1 couple neuronal and glial subtype specification. Cell 109, 61–73 (2002)
Scheffler, B. et al. Phenotypic and functional characterization of adult brain neuropoiesis. Proc. Natl Acad. Sci. USA 102, 9353–9358 (2005)
Novak, A., Guo, C., Yang, W., Nagy, A. & Lobe, C. G. Z/EG, a double reporter mouse line that expresses enhanced green fluorescent protein upon Cre-mediated excision. Genesis 28, 147–155 (2000)
Zappone, M. V. et al. Sox2 regulatory sequences direct expression of a β-geo transgene to telencephalic neural stem cells and precursors of the mouse embryo, revealing regionalization of gene expression in CNS stem cells. Development 127, 2367–2382 (2000)
Parras, C. M. et al. Mash1 specifies neurons and oligodendrocytes in the postnatal brain. EMBO J. 23, 4495–4505 (2004)
Long, J. E. et al. Dlx-dependent and -independent regulation of olfactory bulb interneuron differentiation. J. Neurosci. 27, 3230–3243 (2007)
Bernstein, B. E. et al. A bivalent chromatin structure marks key developmental genes in embryonic stem cells. Cell 125, 315–326 (2006)
Milne, T. A. et al. MLL targets SET domain methyltransferase activity to Hox gene promoters. Mol. Cell 10, 1107–1117 (2002)
Lee, M. G. et al. Demethylation of H3K27 regulates polycomb recruitment and H2A ubiquitination. Science 318, 447–450 (2007)
Swigut, T. & Wysocka, J. H3K27 demethylases, at long last. Cell 131, 29–32 (2007)
Mikkelsen, T. S. et al. Genome-wide maps of chromatin state in pluripotent and lineage-committed cells. Nature 448, 553–560 (2007)
Wysocka, J. et al. WDR5 associates with histone H3 methylated at K4 and is essential for H3 K4 methylation and vertebrate development. Cell 121, 859–872 (2005)
Acknowledgements
We thank J. Rubenstein for anti-DLX2 antibodies and the pCAG-Dlx2 plasmid, D. Rowitch for anti-OLIG2 antibodies, and Y. Dou and R. Roeder for anti-MLL1 antibodies. This work was supported by the Neurosurgery Research and Education Foundation/American Association of Neurological Surgeons, Sandler Family Foundation, Northern California Institute for Research and Education, and the Clinical and Translational Research Institute at the University of California, San Francisco (D.A.L.), California Institute for Regenerative Medicine New Faculty Award and The Chicago Community Trust Searle Scholar Award (J.W.), and the Goldhirsch Foundation, J.G. Bowes Research Fund, and National Institutes of Health (NIH) 5R37-NS028478 (A.A.-B.).
Author Contributions D.A.L. conceived the project, designed and performed experiments, coordinated collaborations, and wrote the manuscript. Y.-C.H. worked on most experiments, quantified all in vivo data, and helped prepare the figures. T.S. and J.W. performed ChIP experiments, helped analyse data and contributed ideas. A.L.M and P.A.E. provided the Mll1F/F mouse, helped perform preliminary experiments in Mll1+/- mice and contributed ideas. J.M.G.V. provided electron microscopy data and histological interpretation. A.A.-B. contributed ideas, interpreted results and helped write the manuscript. All authors discussed the results and edited the manuscript.
Author information
Authors and Affiliations
Corresponding authors
Supplementary information
Supplementary Figures
This file contains Supplementary Figures 1-9 with Legends (PDF 4861 kb)
Rights and permissions
About this article
Cite this article
Lim, D., Huang, YC., Swigut, T. et al. Chromatin remodelling factor Mll1 is essential for neurogenesis from postnatal neural stem cells. Nature 458, 529–533 (2009). https://doi.org/10.1038/nature07726
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature07726
This article is cited by
-
Transcriptional and epigenetic dysregulation impairs generation of proliferative neural stem and progenitor cells during brain aging
Nature Aging (2024)
-
Epigenetic regulation of human-specific gene expression in the prefrontal cortex
BMC Biology (2023)
-
The role of histone modifications: from neurodevelopment to neurodiseases
Signal Transduction and Targeted Therapy (2022)
-
Dormant state of quiescent neural stem cells links Shank3 mutation to autism development
Molecular Psychiatry (2022)
-
COMPASS and SWI/SNF complexes in development and disease
Nature Reviews Genetics (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.