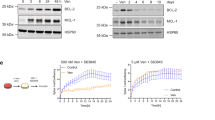
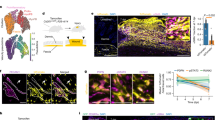
Abstract
Tissue homeostasis and regeneration are regulated by an intricate balance of seemingly competing processes—proliferation versus differentiation, and cell death versus survival1. Here we demonstrate that the loss of epidermal caspase 8, an important mediator of apoptosis2, recapitulates several phases of a wound healing response in the mouse. The epidermal hyperplasia in the caspase 8 null skin is the culmination of signals exchanged between epidermal keratinocytes, dermal fibroblasts and leukocytic cells. This reciprocal interaction is initiated by the paracrine signalling of interleukin 1α (IL1α), which activates both skin stem cell proliferation and cutaneous inflammation. The non-canonical secretion of IL1α is induced by a p38-MAPK-mediated upregulation of NALP3 (also known as NLRP3), leading to inflammasome assembly and caspase 1 activation. Notably, the increased proliferation of basal keratinocytes is counterbalanced by the growth arrest of suprabasal keratinocytes in the stratified epidermis by IL1α-dependent NFκB signalling. Altogether, our findings illustrate how the loss of caspase 8 can affect more than programmed cell death to alter the local microenvironment and elicit processes common to wound repair and many neoplastic skin disorders.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Alonso, L. & Fuchs, E. Stem cells of the skin epithelium. Proc. Natl Acad. Sci. USA 100 (Suppl. 1). 11830–11835 (2003)
Raj, D., Brash, D. E. & Grossman, D. Keratinocyte apoptosis in epidermal development and disease. J. Invest. Dermatol. 126, 243–257 (2006)
Vasioukhin, V., Degenstein, L., Wise, B. & Fuchs, E. The magical touch: genome targeting in epidermal stem cells induced by tamoxifen application to mouse skin. Proc. Natl Acad. Sci. USA 96, 8551–8556 (1999)
Beisner, D. R., Ch’en, I. L., Kolla, R. V., Hoffmann, A. & Hedrick, S. M. Cutting edge: innate immunity conferred by B cells is regulated by caspase-8. J. Immunol. 175, 3469–3473 (2005)
Ito, M. et al. Stem cells in the hair follicle bulge contribute to wound repair but not to homeostasis of the epidermis. Nature Med. 11, 1351–1354 (2005)
Levy, V., Lindon, C., Harfe, B. D. & Morgan, B. A. Distinct stem cell populations regenerate the follicle and interfollicular epidermis. Dev. Cell 9, 855–861 (2005)
Cotsarelis, G. Epithelial stem cells: a folliculocentric view. J. Invest. Dermatol. 126, 1459–1468 (2006)
Martin, P. & Leibovich, S. J. Inflammatory cells during wound repair: the good, the bad and the ugly. Trends Cell Biol. 15, 599–607 (2005)
Jameson, J. & Havran, W. L. Skin γδ T-cell functions in homeostasis and wound healing. Immunol. Rev. 215, 114–122 (2007)
Ichinohe, M. et al. Lack of phospholipase C-δ1 induces skin inflammation. Biochem. Biophys. Res. Commun. 356, 912–918 (2007)
Perez-Moreno, M. et al. p120-catenin mediates inflammatory responses in the skin. Cell 124, 631–644 (2006)
Hobbs, R. M. & Watt, F. M. Regulation of interleukin-1α expression by integrins and epidermal growth factor receptor in keratinocytes from a mouse model of inflammatory skin disease. J. Biol. Chem. 278, 19798–19807 (2003)
Hobbs, R. M., Silva-Vargas, V., Groves, R. & Watt, F. M. Expression of activated MEK1 in differentiating epidermal cells is sufficient to generate hyperproliferative and inflammatory skin lesions. J. Invest. Dermatol. 123, 503–515 (2004)
Murphy, J. E., Morales, R. E., Scott, J. & Kupper, T. S. IL-1α, innate immunity, and skin carcinogenesis: the effect of constitutive expression of IL-1α in epidermis on chemical carcinogenesis. J. Immunol. 170, 5697–5703 (2003)
Werner, S. & Smola, H. Paracrine regulation of keratinocyte proliferation and differentiation. Trends Cell Biol. 11, 143–146 (2001)
Hauser, C., Saurat, J. H., Schmitt, A., Jaunin, F. & Dayer, J. M. Interleukin 1 is present in normal human epidermis. J. Immunol. 136, 3317–3323 (1986)
Kaufman, C. K. & Fuchs, E. It’s got you covered. NF-κB in the epidermis. J. Cell Biol. 149, 999–1004 (2000)
Murphy, J. E., Robert, C. & Kupper, T. S. Interleukin-1 and cutaneous inflammation: a crucial link between innate and acquired immunity. J. Invest. Dermatol. 114, 602–608 (2000)
Yamanaka, K. et al. Skin-specific caspase-1-transgenic mice show cutaneous apoptosis and pre-endotoxin shock condition with a high serum level of IL-18. J. Immunol. 165, 997–1003 (2000)
Groves, R. W., Mizutani, H., Kieffer, J. D. & Kupper, T. S. Inflammatory skin disease in transgenic mice that express high levels of interleukin 1α in basal epidermis. Proc. Natl Acad. Sci. USA 92, 11874–11878 (1995)
Faustin, B. & Reed, J. C. Sunburned skin activates inflammasomes. Trends Cell Biol. 18, 4–8 (2008)
Feldmeyer, L. et al. The inflammasome mediates UVB-induced activation and secretion of interleukin-1β by keratinocytes. Curr. Biol. 17, 1140–1145 (2007)
Johansen, C., Moeller, K., Kragballe, K. & Iversen, L. The activity of caspase-1 is increased in lesional psoriatic epidermis. J. Invest. Dermatol. 127, 2857–2864 (2007)
Al-Mashat, H. A. et al. Diabetes enhances mRNA levels of proapoptotic genes and caspase activity, which contribute to impaired healing. Diabetes 55, 487–495 (2006)
Chun, H. J. et al. Pleiotropic defects in lymphocyte activation caused by caspase-8 mutations lead to human immunodeficiency. Nature 419, 395–399 (2002)
Anderson, J. P. et al. Structural, expression, and evolutionary analysis of mouse CIAS1. Gene 338, 25–34 (2004)
Hohl, D. et al. Characterization of human loricrin. Structure and function of a new class of epidermal cell envelope proteins. J. Biol. Chem. 266, 6626–6636 (1991)
Aravalli, R. N., Hu, S., Rowen, T. N., Palmquist, J. M. & Lokensgard, J. R. Cutting edge: TLR2-mediated proinflammatory cytokine and chemokine production by microglial cells in response to herpes simplex virus. J. Immunol. 175, 4189–4193 (2005)
Kobielak, A. & Fuchs, E. Links between α-catenin, NF-κB, and squamous cell carcinoma in skin. Proc. Natl Acad. Sci. USA 103, 2322–2327 (2006)
Li, J., Yin, H. L. & Yuan, J. Flightless-I regulates proinflammatory caspases by selectively modulating intracellular localization and caspase activity. J. Cell Biol. 181, 321–333 (2008)
Acknowledgements
We thank S. Hedrick, W. Havran, B. Yu, D. Witherden, A. Hoffmann, D. Stachura and members of the Jamora laboratory for providing reagents and helpful discussions. This work was supported by grants from the National Institutes of Health (NIAMS grant number 5R01AR053185-03) and the American Skin Association, and a Career Award from the Dermatology Foundation.
Author Contributions P.L., D.L., C.C., S.C. and C.J. performed the experiments; I.C. engineered the caspase 8 floxed mice; P.L. and C.J. designed the experiments; P.L., D.L. and C.J. wrote the manuscript.
Author information
Authors and Affiliations
Corresponding author
Supplementary information
Supplementary Information
This file contains Supplementary Figures 1-9 with Legends and a Supplementary Reference (PDF 1274 kb)
Rights and permissions
About this article
Cite this article
Lee, P., Lee, DJ., Chan, C. et al. Dynamic expression of epidermal caspase 8 simulates a wound healing response. Nature 458, 519–523 (2009). https://doi.org/10.1038/nature07687
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature07687
This article is cited by
-
Injury-induced interleukin-1 alpha promotes Lgr5 hair follicle stem cells de novo regeneration and proliferation via regulating regenerative microenvironment in mice
Inflammation and Regeneration (2023)
-
A Review on Caspases: Key Regulators of Biological Activities and Apoptosis
Molecular Neurobiology (2023)
-
A systematic summary of survival and death signalling during the life of hair follicle stem cells
Stem Cell Research & Therapy (2021)
-
The role of caspase-8 in the tumor microenvironment of ovarian cancer
Cancer and Metastasis Reviews (2021)
-
Double-stranded RNA induces inflammation via the NF-κB pathway and inflammasome activation in the outer root sheath cells of hair follicles
Scientific Reports (2017)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.