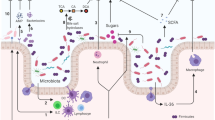
Abstract
Infection with antibiotic-resistant bacteria, such as vancomycin-resistant Enterococcus (VRE), is a dangerous and costly complication of broad-spectrum antibiotic therapy1,2. How antibiotic-mediated elimination of commensal bacteria promotes infection by antibiotic-resistant bacteria is a fertile area for speculation with few defined mechanisms. Here we demonstrate that antibiotic treatment of mice notably downregulates intestinal expression of RegIIIγ (also known as Reg3g), a secreted C-type lectin that kills Gram-positive bacteria, including VRE. Downregulation of RegIIIγ markedly decreases in vivo killing of VRE in the intestine of antibiotic-treated mice. Stimulation of intestinal Toll-like receptor 4 by oral administration of lipopolysaccharide re-induces RegIIIγ, thereby boosting innate immune resistance of antibiotic-treated mice against VRE. Compromised mucosal innate immune defence, as induced by broad-spectrum antibiotic therapy, can be corrected by selectively stimulating mucosal epithelial Toll-like receptors, providing a potential therapeutic approach to reduce colonization and infection by antibiotic-resistant microbes.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Rice, L. B. Emergence of vancomycin-resistant enterococci. Emerg. Infect. Dis. 7, 183–187 (2001)
Rice, L. B. Antimicrobial resistance in gram-positive bacteria. Am. J. Infect. Control 34, S11–S19 (2006)
Murray, B. E. Vancomycin-resistant enterococcal infections. N. Engl. J. Med. 342, 710–721 (2000)
Richards, M. J., Edwards, J. R., Culver, D. H. & Gaynes, R. P. Nosocomial infections in combined medical-surgical intensive care units in the United States. Infect. Control Hosp. Epidemiol. 21, 510–515 (2000)
Wenzel, R. P. & Edmond, M. B. Managing antibiotic resistance. N. Engl. J. Med. 343, 1961–1963 (2000)
Donskey, C. J. The role of the intestinal tract as a reservoir and source for transmission of nosocomial pathogens. Clin. Infect. Dis. 39, 219–226 (2004)
Cash, H. L., Whitham, C. V., Behrendt, C. L. & Hooper, L. V. Symbiotic bacteria direct expression of an intestinal bactericidal lectin. Science 313, 1126–1130 (2006)
Hooper, L. V., Stappenbeck, T. S., Hong, C. V. & Gordon, J. I. Angiogenins: a new class of microbicidal proteins involved in innate immunity. Nature Immunol. 4, 269–273 (2003)
Brandl, K., Plitas, G., Schnabl, B., DeMatteo, R. P. & Pamer, E. G. MyD88-mediated signals induce the bactericidal lectin RegIII gamma and protect mice against intestinal Listeria monocytogenes infection. J. Exp. Med. 204, 1891–1900 (2007)
Cash, H. L., Whitham, C. V. & Hooper, L. V. Refolding, purification, and characterization of human and murine RegIII proteins expressed in Escherichia coli . Protein Expr. Purif. 48, 151–159 (2006)
Donskey, C. J. et al. Effect of antibiotic therapy on the density of vancomycin-resistant enterococci in the stool of colonized patients. N. Engl. J. Med. 343, 1925–1932 (2000)
Miyazaki, S. et al. Development of systemic bacteraemia after oral inoculation of vancomycin-resistant enterococci in mice. J. Med. Microbiol. 50, 695–701 (2001)
Stiefel, U., Pultz, N. J., Helfand, M. S. & Donskey, C. J. Increased susceptibility to vancomycin-resistant Enterococcus intestinal colonization persists after completion of anti-anaerobic antibiotic treatment in mice. Infect. Control Hosp. Epidemiol. 25, 373–379 (2004)
Wells, C. L., Jechorek, R. P. & Erlandsen, S. L. Evidence for the translocation of Enterococcus faecalis across the mouse intestinal tract. J. Infect. Dis. 162, 82–90 (1990)
Wells, C. L., Jechorek, R. P., Maddaus, M. A. & Simmons, R. L. Effects of clindamycin and metronidazole on the intestinal colonization and translocation of enterococci in mice. Antimicrob. Agents Chemother. 32, 1769–1775 (1988)
Sonnenburg, J. L., Chen, C. T. & Gordon, J. I. Genomic and metabolic studies of the impact of probiotics on a model gut symbiont and host. PLoS Biol. 4, e413 (2006)
Pultz, N. J., Stiefel, U., Subramanyan, S., Helfand, M. S. & Donskey, C. J. Mechanisms by which anaerobic microbiota inhibit the establishment in mice of intestinal colonization by vancomycin-resistant Enterococcus . J. Infect. Dis. 191, 949–956 (2005)
Kelly, C. P., Pothoulakis, C. & LaMont, J. T. Clostridium difficile colitis. N. Engl. J. Med. 330, 257–262 (1994)
Kanzler, H., Barrat, F. J., Hessel, E. M. & Coffman, R. L. Therapeutic targeting of innate immunity with Toll-like receptor agonists and antagonists. Nature Med. 13, 552–559 (2007)
Abreu, M. T., Fukata, M. & Arditi, M. TLR signaling in the gut in health and disease. J. Immunol. 174, 4453–4460 (2005)
Lotz, M., Menard, S. & Hornef, M. Innate immune recognition on the intestinal mucosa. Int. J. Med. Microbiol. 297, 379–392 (2007)
Cario, E. et al. Lipopolysaccharide activates distinct signaling pathways in intestinal epithelial cell lines expressing Toll-like receptors. J. Immunol. 164, 966–972 (2000)
Hornef, M. W., Frisan, T., Vandewalle, A., Normark, S. & Richter-Dahlfors, A. Toll-like receptor 4 resides in the Golgi apparatus and colocalizes with internalized lipopolysaccharide in intestinal epithelial cells. J. Exp. Med. 195, 559–570 (2002)
Ortega-Cava, C. F. et al. Strategic compartmentalization of Toll-like receptor 4 in the mouse gut. J. Immunol. 170, 3977–3985 (2003)
Pull, S. L., Doherty, J. M., Mills, J. C., Gordon, J. I. & Stappenbeck, T. S. Activated macrophages are an adaptive element of the colonic epithelial progenitor niche necessary for regenerative responses to injury. Proc. Natl Acad. Sci. USA 102, 99–104 (2005)
Rakoff-Nahoum, S., Paglino, J., Eslami-Varzaneh, F., Edberg, S. & Medzhitov, R. Recognition of commensal microflora by Toll-like receptors is required for intestinal homeostasis. Cell 118, 229–241 (2004)
Nenci, A. et al. Epithelial NEMO links innate immunity to chronic intestinal inflammation. Nature 446, 557–561 (2007)
Hammett-Stabler, C. A. & Johns, T. Laboratory guidelines for monitoring of antimicrobial drugs. Clin. Chem. 44, 1129–1140 (1998)
Acknowledgements
The authors thank L.V. Hooper for providing polyclonal RegIIIγ antiserum, I. Leiner for technical assistance, and W. Falk, B. Salzberger and all members of the Pamer laboratory for discussions. This research was supported by the Alexander von Humboldt Foundation through a Feodor Lynen postdoctoral fellowship to KB and NIH grant AI39031 and AI42135 to EGP.
Author Contributions K.B., G.P., R.P.D. and E.G.P. designed the research. K.B., G.P., C.N.M., C.U., T.J., B.S. and M.F. performed the research. K.B. and E.G.P. analysed the data and wrote the paper.
Author information
Authors and Affiliations
Corresponding author
Supplementary information
Supplementary Information
The file contains Supplementary Figures S1-S8 with Legends. (PDF 271 kb)
Rights and permissions
About this article
Cite this article
Brandl, K., Plitas, G., Mihu, C. et al. Vancomycin-resistant enterococci exploit antibiotic-induced innate immune deficits. Nature 455, 804–807 (2008). https://doi.org/10.1038/nature07250
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature07250
This article is cited by
-
Vitamin B12 produced by Cetobacterium somerae improves host resistance against pathogen infection through strengthening the interactions within gut microbiota
Microbiome (2023)
-
Delayed gut microbiota maturation in the first year of life is a hallmark of pediatric allergic disease
Nature Communications (2023)
-
Reg3γ: current understanding and future therapeutic opportunities in metabolic disease
Experimental & Molecular Medicine (2023)
-
Effects of probiotics (Bacillus coagulans) supplementation after antibiotic administration on growth, immunity, and intestinal microflora in turbot Scophthalmus maximus
Aquaculture International (2023)
-
Effects of rearing system and antibiotic treatment on immune function, gut microbiota and metabolites of broiler chickens
Journal of Animal Science and Biotechnology (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.