Abstract
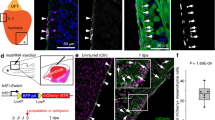
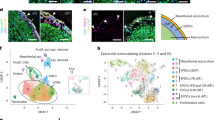
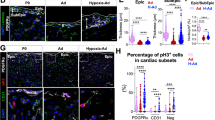
The heart is formed from cardiogenic progenitors expressing the transcription factors Nkx2-5 and Isl1 (refs 1 and 2). These multipotent progenitors give rise to cardiomyocyte, smooth muscle and endothelial cells, the major lineages of the mature heart3,4. Here we identify a novel cardiogenic precursor marked by expression of the transcription factor Wt1 and located within the epicardium—an epithelial sheet overlying the heart. During normal murine heart development, a subset of these Wt1+ precursors differentiated into fully functional cardiomyocytes. Wt1+ proepicardial cells arose from progenitors that express Nkx2-5 and Isl1, suggesting that they share a developmental origin with multipotent Nkx2-5+ and Isl1+ progenitors. These results identify Wt1+ epicardial cells as previously unrecognized cardiomyocyte progenitors, and lay the foundation for future efforts to harness the cardiogenic potential of these progenitors for cardiac regeneration and repair.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Martin-Puig, S., Wang, Z. & Chien, K. R. Lives of a heart cell: tracing the origins of cardiac progenitors. Cell Stem Cell 2, 320–331 (2008)
Laugwitz, K. L., Moretti, A., Caron, L., Nakano, A. & Chien, K. R. Islet1 cardiovascular progenitors: a single source for heart lineages? Development 135, 193–205 (2008)
Moretti, A. et al. Multipotent embryonic Isl1+ progenitor cells lead to cardiac, smooth muscle, and endothelial cell diversification. Cell 127, 1151–1165 (2006)
Wu, S. M. et al. Developmental origin of a bipotential myocardial and smooth muscle cell precursor in the mammalian heart. Cell 127, 1137–1150 (2006)
Manner, J., Perez-Pomares, J. M., Macias, D. & Munoz-Chapuli, R. The origin, formation and developmental significance of the epicardium: a review. Cells Tissues Organs 169, 89–103 (2001)
Wessels, A. & Perez-Pomares, J. M. The epicardium and epicardially derived cells (EPDCs) as cardiac stem cells. Anat. Rec. A 276, 43–57 (2004)
Smart, N. et al. Thymosin β4 induces adult epicardial progenitor mobilization and neovascularization. Nature 445, 177–182 (2007)
Wilm, B., Ipenberg, A., Hastie, N. D., Burch, J. B. & Bader, D. M. The serosal mesothelium is a major source of smooth muscle cells of the gut vasculature. Development 132, 5317–5328 (2005)
Merki, E. et al. Epicardial retinoid X receptor α is required for myocardial growth and coronary artery formation. Proc. Natl Acad. Sci. USA 102, 18455–18460 (2005)
Gittenberger-de Groot, A. C., Vrancken Peeters, M. P., Mentink, M. M., Gourdie, R. G. & Poelmann, R. E. Epicardium-derived cells contribute a novel population to the myocardial wall and the atrioventricular cushions. Circ. Res. 82, 1043–1052 (1998)
Manner, J. Does the subepicardial mesenchyme contribute myocardioblasts to the myocardium of the chick embryo heart? A quail–chick chimera study tracing the fate of the epicardial primordium. Anat. Rec. 255, 212–226 (1999)
Dettman, R. W., Denetclaw, W. J., Ordahl, C. P. & Bristow, J. Common epicardial origin of coronary vascular smooth muscle, perivascular fibroblasts, and intermyocardial fibroblasts in the avian heart. Dev. Biol. 193, 169–181 (1998)
Mikawa, T. & Gourdie, R. G. Pericardial mesoderm generates a population of coronary smooth muscle cells migrating into the heart along with ingrowth of the epicardial organ. Dev. Biol. 174, 221–232 (1996)
Le, Y., Miller, J. L. & Sauer, B. GFPcre fusion vectors with enhanced expression. Anal. Biochem. 270, 334–336 (1999)
Mao, X., Fujiwara, Y. & Orkin, S. H. Improved reporter strain for monitoring Cre recombinase-mediated DNA excisions in mice. Proc. Natl Acad. Sci. USA 96, 5037–5042 (1999)
Vintersten, K. et al. Mouse in red: red fluorescent protein expression in mouse ES cells, embryos, and adult animals. Genesis 40, 241–246 (2004)
Feil, R., Wagner, J., Metzger, D. & Chambon, P. Regulation of Cre recombinase activity by mutated estrogen receptor ligand-binding domains. Biochem. Biophys. Res. Commun. 237, 752–757 (1997)
Stanley, E. G. et al. Efficient Cre-mediated deletion in cardiac progenitor cells conferred by a 3′UTR-ires-Cre allele of the homeobox gene Nkx2–5. Int. J. Dev. Biol. 46, 431–439 (2002)
Moses, K. A., DeMayo, F., Braun, R. M., Reecy, J. L. & Schwartz, R. J. Embryonic expression of an Nkx2–5/Cre gene using ROSA26 reporter mice. Genesis 31, 176–180 (2001)
Rivera-Feliciano, J. et al. Development of heart valves requires Gata4 expression in endothelial-derived cells. Development 133, 3607–3618 (2006)
Heikinheimo, M., Scandrett, J. M. & Wilson, D. B. Localization of transcription factor GATA-4 to regions of the mouse embryo involved in cardiac development. Dev. Biol. 164, 361–373 (1994)
Watt, A. J., Battle, M. A., Li, J. & Duncan, S. A. GATA4 is essential for formation of the proepicardium and regulates cardiogenesis. Proc. Natl Acad. Sci. USA 101, 12573–12578 (2004)
Qyang, Y. et al. The renewal and differentiation of Isl1+ cardiovascular progenitors are controlled by a Wnt/β-Catenin Pathway. Cell Stem Cell 1, 165–179 (2007)
Verzi, M. P., McCulley, D. J., De Val, S., Dodou, E. & Black, B. L. The right ventricle, outflow tract, and ventricular septum comprise a restricted expression domain within the secondary/anterior heart field. Dev. Biol. 287, 134–145 (2005)
Kruithof, B. P. et al. BMP and FGF regulate the differentiation of multipotential pericardial mesoderm into the myocardial or epicardial lineage. Dev. Biol. 295, 507–522 (2006)
Laugwitz, K. L. et al. Postnatal isl1+ cardioblasts enter fully differentiated cardiomyocyte lineages. Nature 433, 647–653 (2005)
Liu, P., Jenkins, N. A. & Copeland, N. G. A highly efficient recombineering-based method for generating conditional knockout mutations. Genome Res. 13, 476–484 (2003)
Rodriguez, C. I. et al. High-efficiency deleter mice show that FLPe is an alternative to Cre-loxP . Nature Genet. 25, 139–140 (2000)
Yang, L. et al. Isl1Cre reveals a common Bmp pathway in heart and limb development. Development 133, 1575–1585 (2006)
Soriano, P. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nature Genet. 21, 70–71 (1999)
Jiao, K. et al. An essential role of Bmp4 in the atrioventricular septation of the mouse heart. Genes Dev. 17, 2362–2367 (2003)
Pu, W. T., Ma, Q. & Izumo, S. NFAT transcription factors are critical survival factors that inhibit cardiomyocyte apoptosis during phenylephrine stimulation in vitro . Circ. Res. 92, 725–731 (2003)
Brent, A. E., Schweitzer, R. & Tabin, C. J. A somitic compartment of tendon progenitors. Cell 113, 235–248 (2003)
Zhou, B. et al. G-CSF-mobilized peripheral blood mononuclear cells from diabetic patients augment neovascularization in ischemic limbs but with impaired capability. J. Thromb. Haemost. 4, 993–1002 (2006)
Gutstein, D. E., Liu, F. Y., Meyers, M. B., Choo, A. & Fishman, G. I. The organization of adherens junctions and desmosomes at the cardiac intercalated disc is independent of gap junctions. J. Cell Sci. 116, 875–885 (2003)
Lobe, C. G. et al. Z/AP, a double reporter for Cre-mediated recombination. Dev. Biol. 208, 281–292 (1999)
Acknowledgements
This work was funded by the National Heart, Lung and Blood Institute of the National Institutes of Health, USA, the American Heart Association, and by a charitable donation from E. P. Marram and K. K. Carpenter. We thank the Schwartz, Harvey, Schneider, Yanagisawa, Evans, Soriano, Orkin and Nagy laboratories for contributing mouse strains used in this study.
Author information
Authors and Affiliations
Corresponding author
Supplementary information
Supplementary Information
This file contains Supplementary Figures 1-12 with Legends and Supplementary Table 1. (PDF 4602 kb)
Supplementary Movie 1
This file contains Supplementary Movie 1 showing spontaneous contraction in Wt1GFPCre/+; Z/Red cells. (MOV 554 kb)
Supplementary Movie 2
This file contains Supplementary Movie 2 showing calcium transients in a Wt1GFPCre/+; Z/Red cell, loaded with the calcium-sensitive indicator Fluo-4 AM. (MOV 1147 kb)
Rights and permissions
About this article
Cite this article
Zhou, B., Ma, Q., Rajagopal, S. et al. Epicardial progenitors contribute to the cardiomyocyte lineage in the developing heart. Nature 454, 109–113 (2008). https://doi.org/10.1038/nature07060
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature07060
This article is cited by
-
Regeneration of the heart: from molecular mechanisms to clinical therapeutics
Military Medical Research (2023)
-
Fetal liver development and implications for liver disease pathogenesis
Nature Reviews Gastroenterology & Hepatology (2023)
-
Cell diversity and plasticity during atrioventricular heart valve EMTs
Nature Communications (2023)
-
Dual genetic tracing reveals a unique fibroblast subpopulation modulating cardiac fibrosis
Nature Genetics (2023)
-
Hypoxia promotes a perinatal-like progenitor state in the adult murine epicardium
Scientific Reports (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.