Abstract
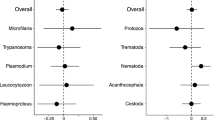
Malaria parasites and related Apicomplexans are the causative agents of the some of the most serious infectious diseases of humans, companion animals, livestock and wildlife. These parasites must undergo sexual reproduction to transmit from vertebrate hosts to vectors, and their sex ratios are consistently female-biased. Sex allocation theory, a cornerstone of evolutionary biology, is remarkably successful at explaining female-biased sex ratios in multicellular taxa, but has proved controversial when applied to malaria parasites. Here we show that, as predicted by theory, sex ratio is an important fitness-determining trait and Plasmodium chabaudi parasites adjust their sex allocation in response to the presence of unrelated conspecifics. This suggests that P. chabaudi parasites use kin discrimination to evaluate the genetic diversity of their infections, and they adjust their behaviour in response to environmental cues. Malaria parasites provide a novel way to test evolutionary theory, and support the generality and power of a darwinian approach.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Charnov, E. L. The Theory of Sex Allocation (Princeton Univ. Press, Princeton, 1982)
Frank, S. A. Sex allocation theory for birds and mammals. Annu. Rev. Ecol. Syst. 21, 13–55 (1990)
Frank, S. A. A touchstone in the study of adaptation. Evolution Int. J. Org. Evolution 56, 2561–2564 (2002)
Hamilton, W. D. Extraordinary sex ratios. Science 156, 477–488 (1967)
Hardy, I. C. W. Sex Ratios: Concepts and Research Methods (Cambridge Univ. Press, Cambridge, UK, 2002)
Ferguson, D. J. P. Toxoplasma gondii and sex: Essential or optional extra. Trends Parasitol. 18, 355–359 (2002)
Ferguson, D. J. P. More on Toxoplasma gondii, sex and premature rejection. Trends Parasitol. 19, 157–158 (2003)
Paul, R. E. L., Ariey, F. & Robert, V. The evolutionary ecology of Plasmodium. Ecol. Lett. 6, 866–880 (2003)
Shutler, D., Bennett, G. F. & Mullie, A. Sex proportions of Haemoproteus blood parasites and local mate competition. Proc. Natl Acad. Sci. USA 92, 6748–6752 (1995)
West, S. A., Reece, S. E. & Read, A. F. Toxoplasma gondii, sex and premature rejection. Trends Parasitol. 19, 155–157 (2003)
Reece, S. E., Duncan, A. B., West, S. A. & Read, A. F. Host cell preference and variable transmission strategies in malaria parasites. Proc. R. Soc. Lond. B 272, 511–517 (2005)
Robert, V. et al. Sex ratio of Plasmodium falciparum gametocytes in inhabitants of Dielmo, Senegal. Parasitology 127, 1–8 (2003)
Paul, R. E. L., Coulson, T. N., Raibaud, A. & Brey, P. T. Sex determination in malaria parasites. Science 287, 128–131 (2000)
Paul, R. E. L., Raibaud, A. & Brey, P. T. Sex ratio adjustment in Plasmodium gallinaceum. Parassitologia 41, 153–158 (1999)
Osgood, S. M., Eisen, R. J. & Schall, J. J. Gametocyte sex ratio of a malaria parasite: Experimental test of heritability. J. Parasitol. 88, 494–498 (2002)
Dye, C. & Godfray, H. C. F. On sex ratio and inbreeding in malaria parasite populations. J. Theor. Biol. 161, 131–134 (1993)
Nee, S., West, S. A. & Read, A. F. Inbreeding and parasite sex ratios. Proc. R. Soc. Lond. B 269, 755–760 (2002)
Read, A. F., Anwar, M., Shutler, D. & Nee, S. Sex allocation and population-structure in malaria and related parasitic protozoa. Proc. R. Soc. Lond. B 260, 359–363 (1995)
West, S. A., Smith, T. G. & Read, A. F. Sex allocation and population structure in apicomplexan (protozoa) parasites. Proc. R. Soc. Lond. B 267, 257–263 (2000)
Fisher, R. A. The Genetical Theory of Natural Selection (Clarendon, Oxford, UK, 1930)
West, S. A., Shuker, D. M. & Sheldon, B. C. Sex-ratio adjustment when relatives interact: A test of constraints on adaptation. Evolution Int. J. Org. Evolution 59, 1211–1228 (2005)
Paul, R. E. L. et al. Mating patterns in malaria parasite populations of Papua New Guinea. Science 269, 1709–1711 (1995)
Conway, D. J. et al. High recombination rate in natural populations of Plasmodium falciparum. Proc. Natl Acad. Sci. USA 96, 4506–4511 (1999)
Walliker, D., Babiker, H. A. & Ranford-Cartwright, L. C. in Malaria: Parasite Biology, Pathogenesis and Protection (ed. Sherman, I.) 235–252 (ASM, Washington DC, 1998)
Read, A. F. et al. Gametocyte sex-ratios as indirect measures of outcrossing rates in malaria. Parasitology 104, 387–395 (1992)
West, S. A., Reece, S. E. & Read, A. F. Evolution of gametocyte sex ratios in malaria and related Apicomplexan (protozoan) parasites. Trends Parasitol. 17, 525–531 (2001)
Read, A. F., Smith, T. G., Nee, S. & West, S. A. in Sex Ratio Handbook (ed. Hardy, I. C. W.) 314–332 (Cambridge Univ. Press, Cambridge, UK, 2002)
Robert, V. et al. Malaria transmission in urban Sub-Saharan Africa. Am. J. Trop. Med. Hyg. 68, 169–176 (2003)
Robert, V. et al. Effect of gametocyte sex ratio on infectivity of Plasmodium falciparum to Anopheles gambiae. Trans. R. Soc. Trop. Med. Hyg. 90, 621–624 (1996)
Schall, J. J. Transmission success of the malaria parasite Plasmodium mexicanum into its vector: Role of gametocyte density and sex ratio. Parasitology 121, 575–580 (2000)
West, S. A., Griffin, A. S., Gardner, A. & Diggle, S. P. Social evolution theory for microorganisms. Nature Rev. Microbiol. 4, 597–607 (2006)
Gardner, A., Reece, S. E. & West, S. A. Even more extreme fertility insurance and the sex ratios of protozoan blood parasites. J. Theor. Biol. 223, 515–521 (2003)
West, S. A., Smith, T. G., Nee, S. & Read, A. F. Fertility insurance and the sex ratios of malaria and related hemospororin blood parasites. J. Parasitol. 88, 258–263 (2002)
Pickering, J., Read, A. F., Guerrero, S. & West, S. A. Sex ratio and virulence in two species of lizard malaria parasites. Evol. Ecol. Res.2 171–184 (2000)
Reece, S. E. & Read, A. F. Malaria sex ratios. Trends Ecol. Evol. 15, 259–260 (2000)
van Dijk, M. R. et al. A central role for p48/45 in malaria parasite male gamete fertility. Cell 104, 153–164 (2001)
Khan, S. M. et al. Proteome analysis of separated male and female gametocytes reveals novel sex-specific Plasmodium biology. Cell 121, 675–687 (2005)
Janse, C. J. et al. In vitro formation of ookinetes and functional maturity of Plasmodium-berghei gametocytes. Parasitology 91, 19–29 (1985)
Drew, D. R. & Reece, S. E. Development of reverse-transcription PCR techniques to analyse the density and sex ratio of gametocytes in genetically diverse Plasmodium chabaudi infections. Mol. Biochem. Parasitol. 156, 199–209 (2007)
Werren, J. H. Sex ratio adaptations to local mate competition in a parasitic wasp. Science 208, 1157–1159 (1980)
Mackinnon, M. J. & Read, A. F. Genetic relationships between parasite virulence and transmission in the rodent malaria Plasmodium chabaudi. Evolution Int. J. Org. Evolution 53, 689–703 (1999)
Razakandrainibe, F. G. et al. “Clonal” population structure of the malaria agent Plasmodium falciparum in high-infection regions. Proc. Natl Acad. Sci. USA 102, 17388–17393 (2005)
Nesse, R. M. & Williams, G. C. Why We Get Sick: The New Science of Darwinian Medicine (Times Books, New York, 1995)
Mehdiabadi, N. J. et al. Kin preference in a social microbe. Nature 442, 881–882 (2006)
Crozier, R. H. Genetic clonal recognition abilities in marine-invertebrates must be maintained by selection for something else. Evolution Int. J. Org. Evolution 40, 1100–1101 (1986)
Frank, S. A. A kin selection model for the evolution of virulence. Proc. R. Soc. Lond. B 250, 195–197 (1992)
Frank, S. A. Kin selection and virulence in the evolution of protocells and parasites. Proc. R. Soc. Lond. B 258, 153–161 (1994)
Herre, E. A. Population structure and the evolution of virulence in nematode parasites of fig wasps. Science 259, 1442–1445 (1993)
Al-Olayan, E. M., Williams, G. T. & Hurd, H. Apoptosis in the malaria protozoan, Plasmodium berghei: A possible mechanism for limiting intensity of infection in the mosquito. Int. J. Parasitol. 32, 1133–1143 (2002)
Rousset, F. & Roze, D. Constraints on the origin and maintenance of genetic kin recognition. Evolution Int. J. Org. Evolution 61, 2320–2330 (2007)
Acknowledgements
We thank A. P. Waters, C. Janse and M. R. van Dijk for the genetically modified parasites, and D. H. Nussey, S. A. West, A. F. Read and A. Buckling for discussions. The Wellcome Trust, NERC, BBSRC and Royal Society provided funding.
Author Contributions S.E.R. conceived and designed the experiments, carried out the fitness consequences experiment, analysed sex ratio data and prepared the manuscript. D.R.D. developed the PCR assays, carried out the sex ratio experiments and data collection. A.G. analysed the fitness data and contributed to discussions and manuscript preparation.
Author information
Authors and Affiliations
Corresponding author
Supplementary information
Supplementray Information
The file contains Supplementary Data with Supplementary Table S1-S5 and additional references. (PDF 167 kb)
Rights and permissions
About this article
Cite this article
Reece, S., Drew, D. & Gardner, A. Sex ratio adjustment and kin discrimination in malaria parasites. Nature 453, 609–614 (2008). https://doi.org/10.1038/nature06954
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nature06954
This article is cited by
-
Evolutionary modelling indicates that mosquito metabolism shapes the life-history strategies of Plasmodium parasites
Nature Communications (2023)
-
Evaluation of sustainable susceptibility to Plasmodium vivax infection among colonized Anopheles darlingi and Anopheles deaneorum
Malaria Journal (2022)
-
How a malaria parasite becomes a male
Nature (2022)
-
Increased investment in gametocytes in asymptomatic Plasmodium falciparum infections in the wet season
BMC Infectious Diseases (2021)
-
Real-time PCR assays for detection and quantification of early P. falciparum gametocyte stages
Scientific Reports (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.