Abstract
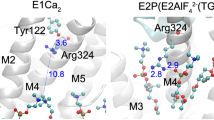
The sarcoplasmic reticulum Ca2+-ATPase, a P-type ATPase, has a critical role in muscle function and metabolism. Here we present functional studies and three new crystal structures of the rabbit skeletal muscle Ca2+-ATPase, representing the phosphoenzyme intermediates associated with Ca2+ binding, Ca2+ translocation and dephosphorylation, that are based on complexes with a functional ATP analogue, beryllium fluoride and aluminium fluoride, respectively. The structures complete the cycle of nucleotide binding and cation transport of Ca2+-ATPase. Phosphorylation of the enzyme triggers the onset of a conformational change that leads to the opening of a luminal exit pathway defined by the transmembrane segments M1 through M6, which represent the canonical membrane domain of P-type pumps. Ca2+ release is promoted by translocation of the M4 helix, exposing Glu 309, Glu 771 and Asn 796 to the lumen. The mechanism explains how P-type ATPases are able to form the steep electrochemical gradients required for key functions in eukaryotic cells.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Rolfe, D. F. & Brown, G. C. Cellular energy utilization and molecular origin of standard metabolic rate in mammals. Physiol. Rev. 77, 731–758 (1997)
Carafoli, E. Calcium signaling: a tale for all seasons. Proc. Natl Acad. Sci. USA 99, 1115–1122 (2002)
Ebashi, S. & Lipmann, F. Adenosine triphosphate-linked concentration of calcium ions in a particulate fraction of rabbit muscle. J. Cell Biol. 14, 389–400 (1962)
Hasselbach, W. Quantitative aspects of the calcium concept of excitation contraction coupling—a critical evaluation. Basic Res. Cardiol. 75, 2–12 (1980)
De Meis, L. The sarcoplasmic reticulum: Transport and Energy Transduction (ed. Bittar, E. E.) (Wiley & Sons, New York, 1981)
Levy, D., Seigneuret, M., Bluzat, A. & Rigaud, J. L. Evidence for proton countertransport by the sarcoplasmic reticulum Ca2+-ATPase during calcium transport in reconstituted proteoliposomes with low ionic permeability. J. Biol. Chem. 265, 19524–19534 (1990)
Cornelius, F. & Moller, J. V. Electrogenic pump current of sarcoplasmic reticulum Ca2+-ATPase reconstituted at high lipid/protein ratio. FEBS Lett. 284, 46–50 (1991)
Yu, X., Carroll, S., Rigaud, J. L. & Inesi, G. H. + countertransport and electrogenicity of the sarcoplasmic reticulum Ca2+ pump in reconstituted proteoliposomes. Biophys. J. 64, 1232–1242 (1993)
Toyoshima, C., Nakasako, M., Nomura, H. & Ogawa, H. Crystal structure of the calcium pump of sarcoplasmic reticulum at 2.6 Å resolution. Nature 405, 647–655 (2000)
Toyoshima, C. & Nomura, H. Structural changes in the calcium pump accompanying the dissociation of calcium. Nature 418, 605–611 (2002)
Sorensen, T. L., Moller, J. V. & Nissen, P. Phosphoryl transfer and calcium ion occlusion in the calcium pump. Science 304, 1672–1675 (2004)
Toyoshima, C. & Mizutani, T. Crystal structure of the calcium pump with a bound ATP analogue. Nature 430, 529–535 (2004)
Toyoshima, C., Nomura, H. & Tsuda, T. Lumenal gating mechanism revealed in calcium pump crystal structures with phosphate analogues. Nature 432, 361–368 (2004)
Olesen, C., Sorensen, T. L., Nielsen, R. C., Moller, J. V. & Nissen, P. Dephosphorylation of the calcium pump coupled to counterion occlusion. Science 306, 2251–2255 (2004)
Jensen, A. M., Sorensen, T. L., Olesen, C., Moller, J. V. & Nissen, P. Modulatory and catalytic modes of ATP binding by the calcium pump. EMBO J. 25, 2305–2314 (2006)
Feher, J. J. & Briggs, F. N. Determinants of calcium loading at steady state in sarcoplasmic reticulum. Biochim. Biophys. Acta 727, 389–402 (1983)
Gerdes, U. & Moller, J. V. The Ca2+ permeability of sarcoplasmic reticulum vesicles. II. Ca2+ efflux in the energized state of the calcium pump. Biochim. Biophys. Acta 734, 191–200 (1983)
Yu, X. & Inesi, G. Variable stoichiometric efficiency of Ca2+ and Sr2+ transport by the sarcoplasmic reticulum ATPase. J. Biol. Chem. 270, 4361–4367 (1995)
Artigas, P. & Gadsby, D. C. Na+/K+-pump ligands modulate gating of palytoxin-induced ion channels. Proc. Natl Acad. Sci. USA 100, 501–505 (2003)
Tanford, C. Translocation pathway in the catalysis of active transport. Proc. Natl Acad. Sci. USA 80, 3701–3705 (1983)
Toyoshima, C. & Inesi, G. Structural basis of ion pumping by Ca2+-ATPase of the sarcoplasmic reticulum. Annu. Rev. Biochem. 73, 269–292 (2004)
Takahashi, M., Kondou, Y. & Toyoshima, C. Interdomain communication in calcium pump as revealed in the crystal structures with transmembrane inhibitors. Proc. Natl Acad. Sci. USA 104, 5800–5805 (2007)
Taylor, J. S. Sarcoplasmic reticulum ATPase catalyzes hydrolysis of adenyl-5′-yl imidodiphosphate. J. Biol. Chem. 256, 9793–9795 (1981)
Meltzer, S. & Berman, M. C. Effects of pH, temperature, and calcium concentration on the stoichiometry of the calcium pump of sarcoplasmic reticulum. J. Biol. Chem. 259, 4244–4253 (1984)
Mahaney, J. E., Thomas, D. D. & Froehlich, J. P. The time-dependent distribution of phosphorylated intermediates in native sarcoplasmic reticulum Ca2+-ATPase from skeletal muscle is not compatible with a linear kinetic model. Biochemistry 43, 4400–4416 (2004)
Skou, J. C. The Na,K-pump. Methods Enzymol. 156, 1–25 (1988)
Läuger, P. in Electrogenic pumps Ch. 8 (Sinauer Associates, Sunderland, Massachusetts, 1991)
Danko, S., Yamasaki, K., Daiho, T. & Suzuki, H. Distinct natures of beryllium fluoride-bound, aluminum fluoride-bound, and magnesium fluoride-bound stable analogues of an ADP-insensitive phosphoenzyme intermediate of sarcoplasmic reticulum Ca2+-ATPase: changes in catalytic and transport sites during phosphoenzyme hydrolysis. J. Biol. Chem. 279, 14991–14998 (2004)
Picard, M., Toyoshima, C. & Champeil, P. Effects of inhibitors on luminal opening of Ca2+ binding sites in an E2P-like complex of the sarcoplasmic reticulum Ca2+-ATPase with Be2+-fluoride. J. Biol. Chem. 281, 3360–3369 (2006)
Moller, J. V. et al. Calcium transport by sarcoplasmic reticulum Ca2+-ATPase. Role of the A domain and its C-terminal link with the transmembrane region. J. Biol. Chem. 277, 38647–38659 (2002)
Daiho, T. et al. Deletions of any single residues in Glu40–Ser48 loop connecting a domain and the first transmembrane helix of sarcoplasmic reticulum Ca2+-ATPase result in almost complete inhibition of conformational transition and hydrolysis of phosphoenzyme intermediate. J. Biol. Chem. 278, 39197–39204 (2003)
Lenoir, G. et al. Functional properties of sarcoplasmic reticulum Ca2+-ATPase after proteolytic cleavage at Leu119–Lys120, close to the A-domain. J. Biol. Chem. 279, 9156–9166 (2004)
Daiho, T., Yamasaki, K., Danko, S. & Suzuki, H. Critical role of Glu40–Ser48 loop linking actuator domain and 1st transmembrane helix of Ca2+-ATPase in Ca2+ deocclusion and release from ADP-insensitive phosphoenzyme. J. Biol. Chem. 282, 34429–34447 (2007)
Andersen, J. P. & Vilsen, B. Structure–function relationships of cation translocation by Ca2+- and Na+, K+-ATPases studied by site-directed mutagenesis. FEBS Lett. 359, 101–106 (1995)
Vilsen, B. & Andersen, J. P. Mutation to the glutamate in the fourth membrane segment of Na+,K+-ATPase and Ca2+-ATPase affects cation binding from both sides of the membrane and destabilizes the occluded enzyme forms. Biochemistry 37, 10961–10971 (1998)
Morth, J. P. et al. Crystal structure of the sodium–potassium pump. Nature doi: 10.1038/nature06419 (this issue)
Reyes, N. & Gadsby, D. C. Ion permeation through the Na+,K+-ATPase. Nature 443, 470–474 (2006)
Wakabayashi, S., Ogurusu, T. & Shigekawa, M. Factors influencing calcium release from the ADP-sensitive phosphoenzyme intermediate of the sarcoplasmic reticulum ATPase. J. Biol. Chem. 261, 9762–9769 (1986)
Apell, H. J. Structure–function relationship in P-type ATPases–a biophysical approach. Rev. Physiol. Biochem. Pharmacol. 150, 1–35 (2003)
Jardetzky, O. Simple allosteric model for membrane pumps. Nature 211, 969–970 (1966)
Vidaver, G. A. Inhibition of parallel flux and augmentation of counter flux shown by transport models not involving a mobile carrier. J. Theor. Biol. 10, 301–306 (1966)
Inesi, G., Ma, H., Lewis, D. & Xu, C. Ca2+ occlusion and gating function of Glu309 in the ADP-fluoroaluminate analog of the Ca2+-ATPase phosphoenzyme intermediate. J. Biol. Chem. 279, 31629–31637 (2004)
Abramson, J. et al. Structure and mechanism of the lactose permease of Escherichia coli . Science 301, 610–615 (2003)
Boudker, O., Ryan, R. M., Yernool, D., Shimamoto, K. & Gouaux, E. Coupling substrate and ion binding to extracellular gate of a sodium-dependent aspartate transporter. Nature 445, 387–393 (2007)
Dawson, R. J. & Locher, K. P. Structure of a bacterial multidrug ABC transporter. Nature 443, 180–185 (2006)
Hvorup, R. N. et al. Asymmetry in the structure of the ABC transporter–binding protein complex BtuCD–BtuF. Science 317, 1387–1390 (2006)
Adams, P. D. et al. PHENIX: building new software for automated crystallographic structure determination. Acta Crystallogr. D 58, 1948–1954 (2002)
Collet, J. F., Stroobant, V. & Van Schaftingen, E. Evidence for phosphotransferases phosphorylated on aspartate residue in N-terminal DXDX(T/V) motif. Methods Enzymol. 354, 177–188 (2002)
Purich, D. L. Use of sodium borohydride to detect acyl-phosphate linkages in enzyme reactions. Methods Enzymol. 354, 168–177 (2002)
Fiske, C. H. & Subbarow, Y. The colorimetric determination of phosphours. J. Biol. Chem. 26, 375–400 (1925)
Andersen, J. P., Lassen, K. & Moller, J. V. Changes in Ca2+ affinity related to conformational transitions in the phosphorylated state of soluble monomeric Ca2+-ATPase from sarcoplasmic reticulum. J. Biol. Chem. 260, 371–380 (1985)
Sorensen, T. L., Olesen, C., Jensen, A. M., Moller, J. V. & Nissen, P. Crystals of sarcoplasmic reticulum Ca2+-ATPase. J. Biotechnol. 124, 704–716 (2006)
Kabsch, W. Automatic processing of rotation diffraction data from crystals of initially unknown symmetry and cell constants. J. Appl. Cryst. 26, 795–800 (1993)
McCoy, A. J., Grosse-Kunstleve, R. W., Storoni, L. C. & Read, R. J. Likelihood-enhanced fast translation functions. Acta Crystallogr. D 61, 458–464 (2005)
Terwilliger, T. C. Maximum-likelihood density modification. Acta Crystallogr. D 56, 965–972 (2000)
Jones, T. A., Zou, J. Y., Cowan, S. W. & Kjeldgaard, M. Improved methods for building protein models in electron density maps and the location of errors in these models. Acta Crystallogr. A 47, 110–119 (1991)
Brunger, A. T. et al. Crystallography & NMR system: A new software suite for macromolecular structure determination. Acta Crystallogr. D 54, 905–921 (1998)
Laskowski, R. A., Moss, D. S. & Thornton, J. M. Main-chain bond lengths and bond angles in protein structures. J. Mol. Biol. 231, 1049–1067 (1993)
Sickmann, A. & Meyer, H. E. Phosphoamino acid analysis. Proteomics 1, 200–206 (2001)
Allegrini, S. et al. Bovine cytosolic 5′-nucleotidase acts through the formation of an aspartate 52-phosphoenzyme intermediate. J. Biol. Chem. 276, 33526–33532 (2001)
Collet, J. F., Stroobant, V. & Van Schaftingen, E. Mechanistic studies of phosphoserine phosphatase, an enzyme related to P-type ATPases. J. Biol. Chem. 274, 33985–33990 (1999)
Sanders, D. A., Gillece-Castro, B. L., Stock, A. M., Burlingame, A. L. & Koshland, D. E. Identification of the site of phosphorylation of the chemotaxis response regulator protein, CheY. J. Biol. Chem. 264, 21770–21778 (1989)
Murphy, A. J. & Coll, R. J. Fluoride is a slow, tight-binding inhibitor of the calcium ATPase of sarcoplasmic reticulum. J. Biol. Chem. 267, 5229–5235 (1992)
Moller, J. V., Lind, K. E. & Andersen, J. P. Enzyme kinetics and substrate stabilization of detergent-solubilized and membraneous (Ca2+ + Mg2+)-activated ATPase from sarcoplasmic reticulum. Effect of protein–protein interactions. J. Biol. Chem. 255, 1912–1920 (1980)
Acknowledgements
We dedicate this paper to the memory of B. Holm. We thank B. Nielsen, M.-B. Hemmingsen and A. M. Nielsen for technical assistance; J. L. Karlsen and F. Fredslund for technical discussions; and D. Flot and L. Gordon at beamlines ID 23-1 and -2 (operated jointly with EMBL-Grenoble) and ID 29 at the European Synchrotron Radiation Facility (ESRF) for help with data collection. Beamtime at the EMBL-DESY synchrotron Hamburg Germany is also acknowledged. This work was supported by the Danish Natural Science Research Council through the DANSYNC program, the Danish Medical Research Council, the Aarhus University Research Foundation, and the Novo Nordisk Foundation. C.Ol. is the recipient of a stipend from the PC Petersen Foundation and a PhD fellowship from the faculty of Health Sciences Aarhus University. A PhD fellowship (A.-M.L.W.) was financed by the Lundbeck Foundation. M.P. was supported by a post-doctoral fellowship from the Federation of European Biochemical Societies (FEBS) and P.N. is supported by a Hallas-Møller stipend of the Novo Nordisk Foundation.
Author Contributions C.Ol., M.P., A.-M.L.W., J.V.M. and P.N. contributed equally to this work. J.P.M. assisted with data collection and structure determination. C.Ox. and C.G. contributed with mass-spectrometry data and analysis.
Author information
Authors and Affiliations
Corresponding authors
Supplementary information
Supplementary Information
The file contains Supplementary Table 1, Supplementary Figures S1-S6 with Legends and additional references. (PDF 1784 kb)
Supplementary Movie
The file contains Supplementary Movie 1. The movie shows the structure of the α β γ-complex of the Na,K-ATPase from pig kidney rotating (α-subunit in blue, β in wheat, γ in red, C-terminal switch in pink, and two Rb+ ions in magenta. The β ectodomain is represented by the experimental electron density (MOV 3297 kb)
Rights and permissions
About this article
Cite this article
Olesen, C., Picard, M., Winther, AM. et al. The structural basis of calcium transport by the calcium pump. Nature 450, 1036–1042 (2007). https://doi.org/10.1038/nature06418
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nature06418
This article is cited by
-
Identification and characterisation of spontaneous mutations causing deafness from a targeted knockout programme
BMC Biology (2022)
-
Electrostatic interactions between single arginine and phospholipids modulate physiological properties of sarcoplasmic reticulum Ca2+-ATPase
Scientific Reports (2022)
-
Structural basis for gating mechanism of the human sodium-potassium pump
Nature Communications (2022)
-
The SarcoEndoplasmic Reticulum Calcium ATPase (SERCA) pump: a potential target for intervention in aging and skeletal muscle pathologies
Skeletal Muscle (2021)
-
Targeting oncogenic Notch signaling with SERCA inhibitors
Journal of Hematology & Oncology (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.