Abstract
Arising from: D. H. Jones et al. Nature 440, 692–696 (2006)10.1038/nature04524; Jones et al. reply
The RANK/RANKL signalling mechanism is the final common pathway of osteoclast formation and activity1. Inhibitors of RANK ligand (RANKL) that bind to RANK (for 'receptor activator of NF-κB'), such as osteoprotegerin (OPG), neutralizing antibodies against RANKL and soluble RANK antagonists, are well described inhibitors of bone metastasis in preclinical and clinical models, presumably because of their effects on osteoclasts2. Jones et al.3 show that OPG inhibits bone metastasis after intracardiac injection of B16F10 murine melanoma cells, but claim that bone metastases are entirely independent of osteoclast formation and bone resorption: rather, they are caused by an effect on cell migration through RANK. However, we question whether these surprising conclusions are rigorously supported by their data3.
Similar content being viewed by others
Main
The significance of RANK production by cancer cells has long been of interest, particularly as this TNF (for tumour-necrosis factor)-receptor family member is expressed in several breast-cancer cell lines and in a series of clinical breast cancers4. However, its role, if any, in the bone metastatic process is difficult to unravel in the absence of osteoclastic bone resorption. With this preclinical model of bone metastasis5, tumour burden at bone metastatic sites is decreased whenever osteoclasts are inhibited — whether by bisphosphonates, inhibition of RANKL, neutralizing antibodies against parathyroid-hormone-related protein (PTHrP) or inhibitors of PTHrP transcription2,6,7. Evidence for a central role for osteoclasts in bone metastasis is therefore compelling.
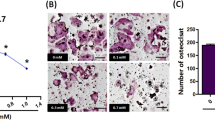
Jones et al.3 assume that osteoclasts have no role in their model of metastasis. To prove that, quantitative histology would be required, with the number of osteoclasts counted from the early stages of tumour-cell growth in bone. Once bone tumours have become large and generally invasive (which includes cell migration), it becomes difficult to see and quantify osteoclasts, even though classical large resorption-site Howship's lacunae are present. These lacunae are characteristic of osteoclasts and cannot be generated by any other cell, as shown in a scanning electron microscope study of human cancers metastasized to bone8. Jones et al.3 indicate (their data are not shown) that treatment with the bisphosphonate zoledronic acid is ineffective in preventing bone-tumour growth. However, they do not provide the doses used, the timing of treatment, evidence that the zoledronic acid can inhibit osteoclasts, details of the behaviour of appropriate tumour controls (such as breast-cancer cells), or indication of whether treatment with OPG in the same experiment is effective.
Investigations into the role of the bone microenvironment in helping cancers to establish and grow in bone indicate that the attachment, maturation and activation of osteoclast precursors are likely to be important for tumour expansion, in which case the RANKL/RANK signalling system would be a suitable target for prevention and treatment. If the additional role of RANK-mediated cell motility proposed by Jones et al.3 does indeed replace that of osteoclasts in the case of B16F10 melanoma cells, the provision of more convincing evidence would dispel any confusion.
References
Teitelbaum, S. L. & Ross, F. P. Nature Rev. Genet. 4, 638–649 (2003).
Mundy, G. R. Nature Rev. Cancer 2, 584–593 (2002).
Jones, D. H. et al. Nature 440, 692–696 (2006).
Thomas, R. J. et al. Endocrinology 140, 4451–4458 (1999).
Arguello, F., Baggs, R. B. & Frantz, C. N. Cancer Res. 48, 6876–6881 (1988).
Guise, T. A. et al. J. Clin. Invest. 98, 1544–1549 (1996).
Gallwitz, W. E., Guise, T. A. & Mundy, G. R. J. Clin. Invest. 110, 1559–1572 (2002).
Boyde, A., Maconacchie, E., Reid, S. A., Delling, G. & Mundy, G. R. Scanning Electron. Micros. IV, 1537–1554 (1986).
Author information
Authors and Affiliations
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Martin, T., Mundy, G. Can osteoclasts be excluded?. Nature 445, E19 (2007). https://doi.org/10.1038/nature05657
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature05657
This article is cited by
-
Extracellular vesicles from human urine-derived stem cells prevent osteoporosis by transferring CTHRC1 and OPG
Bone Research (2019)
-
RANK-Fc inhibits malignancy via inhibiting ERK activation and evoking caspase-3-mediated anoikis in human osteosarcoma cells
Clinical & Experimental Metastasis (2010)
-
Can osteoclasts be excluded? (Reply)
Nature (2007)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.