Abstract
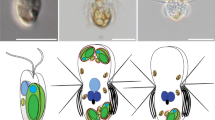
It is well documented that organelles can be retained and used by predatory organisms, but in most cases such sequestrations are limited to plastids of algal prey1. Furthermore, sequestrations of prey organelles are typically highly ephemeral2 as a result of the inability of the organelle to remain functional in the absence of numerous nuclear-encoded genes involved in its regulation, division and function3. The marine photosynthetic ciliate Myrionecta rubra (Lohmann 1908) Jankowski 1976 (the same as Mesodinium rubrum)4 is known to possess organelles of cryptophyte origin5,6,7,8,9, which has led to debate concerning their status as permanent symbiotic or temporary sequestered fixtures5,6,7,8,9,10,11,12,13. Recently, M. rubra has been shown to steal plastids (that is, chloroplasts) from the cryptomonad, Geminigera cryophila, and prey nuclei were observed to accumulate after feeding10. Here we show that cryptophyte nuclei in M. rubra are retained for up to 30 days, are transcriptionally active and service plastids derived from multiple cryptophyte cells. Expression of a cryptophyte nuclear-encoded gene involved in plastid function declined in M. rubra as the sequestered nuclei disappeared from the population. Cytokinesis, plastid performance and their replication are dependent on recurrent stealing of cryptophyte nuclei. Karyoklepty (from Greek karydi, kernel; kleftis, thief) represents a previously unknown evolutionary strategy for acquiring biochemical potential.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Blackbourn, D. J., Taylor, F. J. R. & Blackbourn, J. Foreign organelle retention by ciliates. J. Protozool. 20, 286–288 (1973)
Stoecker, D. K. & Silver, M. W. Replacement of aging chloroplasts in Strombidium capitatum (Ciliophora: Oligotrichida). Mar. Biol. 107, 491–502 (1990)
Martin, W. et al. Gene transfer to the nucleus and the evolution of chloroplasts. Nature 393, 162–165 (1998)
Lynn, D. H. & Small, E. B. In Illustrated Guide to the Protozoa (eds Lee, J. J., Leedale, G. F. & Bradbury, P.) 477–478 (Society of Protozoologists, Lawrence, Kansas, 2000)
Taylor, F. J. R., Blackbourn, D. J. & Blackbourn, J. The red-water ciliate Mesodinium rubrum and its ‘incomplete symbionts’: a review including new ultrastructural observations. J. Fish. Res. Bd Can. 28, 391–407 (1971)
Lindholm, T. Mesodinium rubrum—a unique photosynthetic ciliate. Adv. Aquat. Microbiol. 3, 1–48 (1985)
Taylor, F. J. R., Blackbourn, D. J. & Blackbourn, J. Ultrastructure of the chloroplasts and associated structures within the marine ciliate Mesodinium rubrum (Lohmann). Nature 224, 819–821 (1969)
Hibberd, D. J. Observations on the ultrastructure of the cryptomonad endosymbiont of the red water ciliate Mesodinium rubrum. J. Mar. Biol. Assoc. UK 57, 45–61 (1977)
Oakley, B. R. & Taylor, F. J. R. Evidence for a new type of endosymbiotic organization in a population of the ciliate Mesodinium rubrum from British Columbia. Biosystems 10, 361–369 (1978)
Gustafson, D. E., Stoecker, D. K., Johnson, M. D., Van Heukelem, W. F. & Sneider, K. Cryptophyte algae are robbed of their organelles by the marine ciliate Mesodinium rubrum. Nature 405, 1049–1052 (2000)
Johnson, M. D. & Stoecker, D. K. The role of feeding in growth and the photophysiology of Myrionecta rubra. Aquat. Microb. Ecol. 39, 303–312 (2005)
Johnson, M. D., Tengs, T., Oldach, D. & Stoecker, D. K. Sequestration, performance and functional control of cryptophyte plastids in the ciliate Myrionecta rubra (Ciliophora). J. Phycol. 42, 1235–1246 (2006)
Hansen, P. J. & Fenchel, T. The bloom-forming ciliate Mesodinium rubrum harbours a single permanent endosymbiont. Mar. Biol. Res. 2, 169–177 (2006)
Johnson, M. D., Tengs, T., Oldach, D. W., Delwiche, C. F. & Stoecker, D. K. Highly divergent SSU rRNA genes found in the marine ciliates Myrionecta rubra and Mesodinium pulex. Protist 155, 347–359 (2004)
Deane, J. A. et al. Evidence for nucleomorph to host nucleus gene transfer: light-harvesting complex proteins from cryptomonads and chlorarachniophytes. Protist 151, 239–252 (2000)
Livak, K. J. & Schmittgen, T. D. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods 25, 402–408 (2001)
Gillott, M. A. & Gibbs, S. P. The cryptophyte nucleomorph: its ultrastructure and evolutionary significance. J. Phycol. 16, 558–568 (1980)
Goff, L. J. & Coleman, A. W. Fate of parasite and host organelle DNA during cellular transformation of red algae by their parasites. Plant Cell 7, 1899–1911 (1995)
Wilcox, L. W. & Wedemayer, G. J. Gymnodinium acidotum Nygaard (Pyrrophyta), a dinoflagellate with an endosymbiotic cryptomonad. J. Phycol. 20, 236–242 (1984)
Fields, S. D. & Rhodes, R. G. Ingestion and retention of Chroomonas spp. (Cryptophyceae) by Gymnodinium acidotum (Dinophyceae). J. Phycol. 27, 525–529 (1991)
Gast, R. J., Moran, D. M., Dennett, M. R. & Caron, D. A. Kleptoplastidy in an Antarctic dinoflagellate: caught in evolutionary transition? Environ. Microb. advance online publication, doi:10.1111/j.1462-2920.2006.01109.x (7 August, 2006)
Maddison, W. P. & Maddison, D. R. MacClade—Analysis of Phylogeny and Character Evolution (Sinauer, Sunderland, Massachusetts, 1992)
Miller, P. E. & Scholin, C. A. Identification and enumeration of cultured and wild Pseudo-nitzschia (Bacillariophyceae) using species-specific LSU rRNA-targeted fluorescent probes and filter-based whole cell hybridization. J. Phycol. 34, 371–382 (1998)
Saldarriaga, J. F., McEwan, M. L., Fast, N. M., Taylor, F. J. R. & Keeling, P. J. Multiple protein phylogenies show that Oxyrrhis marina and Perkinsus marinus are early branches of the dinoflagellate lineage. Int. J. Syst. Evol. Microbiol. 53, 355–365 (2003)
Acknowledgements
We thank T. Kana, D.W. Coats, K. Bidle and P. Falkowski for comments on this manuscript; D. Gustafson, S. Heyward and H. Bowers for advice and/or technical assistance; T. Kana for use of his PAM fluorimeter; and C. Scholin for assistance with FISH protocols. This project was funded by a National Science Foundation grant (to D.K.S.).
Author Contributions M.D.J. and D.K.S. conceived of the project. M.D.J. conducted all laboratory experiments and data analysis for the paper. D.K.S., D.O. and C.F.D. provided methodological expertise and contributed to the interpretation of data. M.D.J. wrote most of the paper, with contributions and advice from D.K.S., D.O. and C.F.D.
The sequences for the β-tubulin gene, CbbX, LHCC10 and psbA are deposited in GenBank under accession numbers EF151014, EF151015, EF151016 and EF151017.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The sequences for the β-tubulin gene, CbbX, LHCC10 and psbA are deposited in GenBank under accession numbers EF151014, EF151015, EF151016 and EF151017. Reprints and permissions information is available at www.nature.com/reprints. The authors declare no competing financial interests.
Supplementary information
Supplementary Information
This file contains Supplementary Methods, Supplementary Figures 1-4 with Legends and Supplementary Table 1. (PDF 170 kb)
Rights and permissions
About this article
Cite this article
Johnson, M., Oldach, D., Delwiche, C. et al. Retention of transcriptionally active cryptophyte nuclei by the ciliate Myrionecta rubra. Nature 445, 426–428 (2007). https://doi.org/10.1038/nature05496
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nature05496
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.