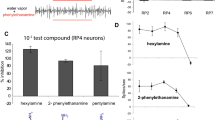
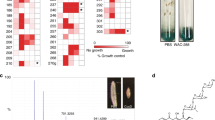
Abstract
Insects transmit disease to hundreds of millions of people a year, and cause enormous losses to the world's agricultural output. Many insects find the human or plant hosts on which they feed, and identify and locate their mates, primarily through olfaction and taste. Major advances have recently been made in understanding insect chemosensation at the molecular and cellular levels. These advances have provided new opportunities to control insects that cause massive damage to health and agriculture across the world.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Snow, R. W., Guerra, A. A., Noor, A. M., Myint, H. Y. & Hay, S. I. The global distribution of clinical episodes of Plasmodium falciparum malaria. Nature 434, 214–217 (2005).
WHO & UNICEF World Malaria Report 2005 [online] <http://www.rbm.who.int/wmr2005> (2005).
Smallegange, R. C., Qiu, Y. T., Van Loon, J. J. A. & Takken, W. Synergism between ammonia, lactic acid and carboxylic acids as kairomones in the host-seeking behaviour of the malaria mosquito Anopheles gambiae sensu stricto (Diptera: Culicidae). Chem. Senses 30, 145–152 (2005).
Qiu, Y. T., Smallegange, R. C., van Loon, J. J. A. & Takken, W. Interindividual variation in odour-mediated attractiveness of human odours to the malaria mosquito Anopheles gambiae. Med. Vet. Entomol. 20, 280–287 (2006).
Schreck, C. E., Kline, D. L. & Carlson, D. A. Mosquito attraction to substances from the skin of different humans. J. Am. Mosquito Control Assoc. 6, 406–410 (1990).
White, G. B. Anopheles gambiae complex and disease transmission in Africa. Trans. R. Soc. Trop. Med. Hyg. 68, 278–299 (1974).
Pates, H. V., Takken, W., Stuke, K. & Curtis, C. F. Differential behaviour of Anopheles gambiae sensu stricto (Diptera: Culicidae) to human and cow odours in the laboratory. Bull. Entomol. Res. 91, 289–296 (2001).
Pimentel, D. Diversification of biological control strategies in agriculture. Crop Protection 10, 243–253 (1991).
Haney, P. B. in Boll Weevil Eradication in the United States through 1999 (eds Dickerson, W. A. et al.) 7–24 (The Cotton Foundation Publisher, Memphis, 2001).
Harris, K. F. & Maramorosch, K. (eds) Vectors of Plant Pathogens (Academic, New York, 1980).
Judd, J. G. R. & Borden, J. Distant olfactory response of the onion fly, Delia antiqua, to host-plant odour in the field. Physiol. Entomol. 14, 429–441 (1989).
Zhang, A. et al. Identification of a new blend of apple volatiles attractive to the apple maggot, Rhagoletis pomonella. J. Chem. Ecol. 25, 1221–1231 (1999).
Glendinning, J. I., Davis, A. & Ramaswamy, S. Contribution of different taste cells and signaling pathways to the discrimination of 'bitter' taste stimuli by an insect. J. Neurosci. 22, 7281–7287 (2002).
Del Campo, M. L. et al. Host recognition by the tobacco hornworm is mediated by a host plant compound. Nature 411, 186–189 (2001).
Fraenkel, G. S. The raison d'être of secondary plant substances. Science 129, 1466–1470 (1959).
Metcalf, R. L., Metcalf, R. A. & Rhodes, A. M. Cucurbitacins as kairomones for diabroticite beetles. Proc. Natl Acad. Sci. USA 77, 3769–3772 (1980).
Witzgall, P., Lindblom, T., Bengtsson, M. & Tóth, M. The Pherolist Phero Net [online] <http://www-pherolist.slu.se./pherolist.php> (2004).
Mustaparta, H. in Insect Pheromone Research: New Directions (eds Cardé, R. T. & Minks, A. K.) 144–163 (Chapman & Hall, New York, 1997).
Butenandt, A., Beckmann, R., Stamm, D. & Hecker, E. Uber den Sexual-Lockstoff des Seidenspinners Bombyx mori. Reindarstellung und Konstitution. Z. Naturforsch. 14, 283–284 (1959).
Shields, V. D. C. & Hildebrand, J. G. Responses of a population of antennal olfactory receptor cells in the female moth Manduca sexta to plant-associated volatile organic compounds. J. Comp. Physiol. A 186, 1135–1151 (2001).
De Bruyne, M., Clyne, P. J. & Carlson, J. R. Odor coding in a model olfactory organ: the Drosophila maxillary palp. J. Neurosci. 19, 4520–4532 (1999).
Vogt, R. G. & Riddiford, L. M. Pheromone binding and inactivation by moth antennae. Nature 293, 161–163 (1981).
Sandler, B. H., Nikonova, L., Leal, W. S. & Clardy, J. Sexual attraction in the silkworm moth: structure of the pheromone-binding protein–bombykol complex. Chem. Biol. 7, 143–151 (2000).
Horst, R. et al. NMR structure reveals intramolecular regulation mechanism for pheromone binding and release. Proc. Natl Acad. Sci. USA 98, 14374–14379 (2001).
Campanacci, V. et al. Revisiting the specificity of Mamestra brassicae and Antheraea polyphemus pheromone-binding proteins with a fluorescence binding assay. J. Biol. Chem. 276, 20078–20084 (2001).
Maida, R., Krieger, J., Gebauer, T., Lange, U. & Ziegelberger, G. Three pheromone-binding proteins in olfactory sensilla of the two silkmoth species Antheraea polyphemus and Antheraea pernyi. Eur. J. Biochem. 267, 2899–2908 (2000).
Kaissling, K.-E. Olfactory perireceptor and receptor events in moths: a kinetic model. Chem. Senses 26, 125–150 (2001).
Leal, W. S. Pheromone reception. Top. Curr. Chem. 240, 1–36 (2004).
Schoonhoven, L. M., Van Loon, J. J. A. & Dicke, M. Insect–Plant Biology (Oxford Univ. Press, UK, Oxford, 2005).
Schoonhoven, L. M., Blaney, W. M. & Simmonds, M. S. J. in Insect–Plant Interactions (ed. Bernays, E. A.) 59–79 (CRC, Boca Raton, 1992).
Mitchell, B. K. Physiology of an ATP receptor in labellar sensilla of the tsetse fly Glossina morsitans morsitans Westw. (Diptera: Glossinidae). J. Exp. Biol. 65, 259–271 (1976).
Van der Goes van Naters, W. M. & Rinkes, T. H. N. Taste stimuli for tsetse flies on the human skin. Chem. Senses 18, 437–444 (1993).
Van der Goes van Naters, W. M. & Den Otter, C. J. Amino acids as taste stimuli for tsetse flies. Physiol. Entomol. 23, 278–284 (1998).
Gilbert, I. H., Gouck, H. K. & Smith, C. N. New mosquito repellents. J. Econ. Entomol. 48, 741–743 (1955).
Leak, S. G. A. Tsetse Biology and Ecology: Their Role in the Epidemiology and Control of Trypanosomosis (CABI, Wallingford, 1999).
Kuhar, T. P., Mori, K. & Dickens, J. C. Potential of a synthetic aggregation pheromone for integrated pest management of Colorado potato beetle. Agric. For. Entomol. 77–81 (2006).
Arn, H. & Louis, F. in Insect Pheromone Research: New Directions (eds Cardé, R. T. & Minks, A. K.) 377–382 (Chapman & Hall, New York, 1997).
Schmutterer, H. Properties and potential of natural pesticides from the neem tree, Azadirachta indica. Annu. Rev. Entomol. 35, 271–297 (1990).
Koul, O. Insect Antifeedants (CRC, Boca Raton, 2005).
Klun, J. A. et al. Comparative resistance of Anopheles albimanus and Aedes aegypti to N,N-diethyl-3-methylbenzamide (Deet) and 2-methylpiperidinyl-3-cyclohexen-1-carboxamide (AI3-37220) in laboratory human-volunteer repellent assays. J. Med. Entomol. 41, 418–422 (2004).
Mota-Sanchez, D., Hollingworth, R. M., Grafius, E. J. & Moyer, D. D. Resistance and cross-resistance to neonicotinoid insecticides and spinosad in the Colorado potato beetle, Leptinotarsa decemlineata (Say) (Coleoptera: Chrysomelidae). Pest Manag. Sci. 62, 30–37 (2006).
Glare, T. R. & O'Callaghan, M. Bacillus thuringiensis: Biology, Ecology and Safety (John Wiley & Sons, Chichester, 2000).
Hajek, A. Natural Enemies: An Introduction to Biological Control (Cambridge Univ. Press, Cambridge, UK, 2004).
Clyne, P. J. et al. A novel family of divergent seven-transmembrane proteins: candidate odorant receptors in Drosophila. Neuron 22, 327–338 (1999).
Vosshall, L. B., Amrein, H., Morozov, P. S., Rzhetsky, A. & Axel, R. A spatial map of olfactory receptor expression in the Drosophila antenna. Cell 96, 725–736 (1999).
Robertson, H. M., Warr, C. G. & Carlson, J. R. Molecular evolution of the insect chemoreceptor gene superfamily in Drosophila melanogaster. Proc. Natl Acad. Sci. USA 100, 14537–14542 (2003).
Stortkuhl, K. F. & Kettler, R. Functional analysis of an olfactory receptor in Drosophila melanogaster. Proc. Natl Acad. Sci. USA 98, 9381–9385 (2001).
Wetzel, C. H. et al. Functional expression and characterization of a Drosophila odorant receptor in a heterologous cell system. Proc. Natl Acad. Sci. USA 98, 9377–9380 (2001).
Dobritsa, A. A., Van der Goes van Naters, W., Warr, C. G., Steinbrecht, R. A. & Carlson, J. R. Integrating the molecular and cellular basis of odor coding in the Drosophila antenna. Neuron 37, 827–841 (2003).
Hallem, E. A., Ho, M. G. & Carlson, J. R. The molecular basis of odor coding in the Drosophila antenna. Cell 117, 965–979 (2004).
Kreher, S. A., Kwon, J. Y. & Carlson, J. R. The molecular basis of odor coding in the Drosophila larva. Neuron 46, 445–456 (2005).
Goldman, A. L., Van der Goes van Naters, W., Lessing, D., Warr, C. G. & Carlson, J. R. Coexpression of two functional odor receptors in one neuron. Neuron 45, 661–666 (2005).
Hallem, E. A. & Carlson, J. R. Coding of odors by a receptor repertoire. Cell 125, 143–160 (2006).
Fox, A. N., Pitts, R. J., Robertson, H. M., Carlson, J. R. & Zwiebel, L. J. Candidate odorant receptors from the malaria vector mosquito Anopheles gambiae and evidence of down-regulation in response to blood feeding. Proc. Natl Acad. Sci. USA 98, 14693–14697 (2001).
Hill, C. A. et al. G protein-coupled receptors in Anopheles gambiae. Science 298, 176–178 (2002).
Krieger, J. et al. A divergent gene family encoding candidate olfactory receptors of the moth Heliothis virescens. Eur. J. Neurosci. 16, 619–628 (2002).
Sakurai, T. et al. Identification and functional characterization of a sex pheromone receptor in the silkmoth Bombyx mori. Proc. Natl Acad. Sci. USA 101, 16653–16658 (2004).
Nakagawa, T., Sakurai, T., Nishioka, T. & Touhara, K. Insect sex-pheromone signals mediated by specific combinations of olfactory receptors. Science 307, 1638–1642 (2005).
Jones, W. D., Nguyen, T. A., Kloss, B., Lee, K. J. & Vosshall, L. B. Functional conservation of an insect odorant receptor gene across 250 million years of evolution. Curr. Biol. 15, R119–R121 (2005).
Krieger, J., Klink, O., Mohl, C., Raming, K. & Breer, H. A candidate olfactory receptor subtype highly conserved across different insect orders. J. Comp. Physiol. A 189, 519–526 (2003).
Neuhaus, E. M. et al. Odorant receptor heterodimerization in the olfactory system of Drosophila melanogaster. Nature Neurosci. 8, 15–17 (2005).
Larsson, M. C. et al. Or83b encodes a broadly expressed odorant receptor essential for Drosophila olfaction. Neuron 43, 703–714 (2004).
Clyne, P. J., Warr, C. G. & Carlson, J. R. Candidate taste receptors in Drosophila. Science 287, 1830–1834 (2000).
Dahanukar, A., Foster, K., Van der Goes van Naters, W. M. & Carlson, J. R. A Gr receptor is required for response to the sugar trehalose in taste neurons of Drosophila. Nature Neurosci. 4, 1182–1186 (2001).
Scott, K. et al. A chemosensory gene family encoding candidate gustatory and olfactory receptors in Drosophila. Cell 104, 661–673 (2001).
Dunipace, L., Meister, S., McNealy, C. & Amrein, H. Spatially restricted expression of candidate taste receptors in the Drosophila gustatory system. Curr. Biol. 11, 822–835 (2001).
Bray, S. & Amrein, H. A putative Drosophila pheromone receptor expressed in male-specific taste neurons is required for efficient courtship. Neuron 39, 1019–1029 (2003).
Wang, Z., Singhvi, A., Kong, P. & Scott, K. Taste representations in the Drosophila brain. Cell 117, 981–991 (2004).
Thorne, N., Chromey, C., Bray, S. & Amrein, H. Taste perception and coding in Drosophila. Curr. Biol. 14, 1065–1079 (2004).
Vogt, R. G., Callahan, F. E., Rogers, M. E. & Dickens, J. C. Odorant binding protein diversity and distribution among the insect orders, as indicated by LAP, an OBP-related protein of the true bug Lygus lineolaris (Hemiptera, Heteroptera). Chem. Senses 24, 481–495 (1999).
Hekmat-Scafe, D. S., Scafe, C. R., McKinney, A. J. & Tanouye, M. A. Genome-wide analysis of the odorant-binding protein gene family in Drosophila melanogaster. Genome Res. 12, 1357–1369 (2002).
Xu, P. X., Zwiebel, L. J. & Smith, D. P. Identification of a distinct family of genes encoding atypical odorant-binding proteins in the malaria vector mosquito, Anopheles gambiae. Insect Mol. Biol. 12, 549–560 (2003).
Araneda, R. C., Kini, A. D. & Firestein, S. The molecular receptive range of an odorant receptor. Nature Neurosci. 3, 1248–1255 (2000).
Oka, Y., Omura, M., Kataoka, H. & Touhara, K. Olfactory receptor antagonism between odorants. EMBO J. 23, 120–126 (2004).
Spehr, M. et al. Identification of a testicular odorant receptor mediating human sperm chemotaxis. Science 299, 2054–2058 (2003).
Hallem, E. A., Fox, A. N., Zwiebel, L. J. & Carlson, J. R. Olfaction: mosquito receptor for human-sweat odorant. Nature 427, 212–213 (2004).
Xu, P., Atkinson, R., Jones, D. N. & Smith, D. P. Drosophila OBP LUSH is required for activity of pheromone-sensitive neurons. Neuron 45, 193–200 (2005).
Maibeche-Coisne, M., Nikonov, A. A., Ishida, Y., Jacquin-Joly, E. & Leal, W. S. Pheromone anosmia in a scarab beetle induced by in vivo inhibition of a pheromone-degrading enzyme. Proc. Natl Acad. Sci. USA 101, 11459–11464 (2004).
De Bruyne, M., Foster, K. & Carlson, J. R. Odor coding in the Drosophila antenna. Neuron 30, 537–552 (2001).
Acknowledgements
Our research is funded by the NIH, a Senior Scholar Award from the Ellison Medical Foundation (J.R.C.) and a grant from the Foundation for the NIH through the Grand Challenges in Global Health initiative.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Additional information
Author Information Reprints and permissions information is available at npg.nature.com/reprintsandpermissions.
Rights and permissions
About this article
Cite this article
van der Goes van Naters, W., Carlson, J. Insects as chemosensors of humans and crops. Nature 444, 302–307 (2006). https://doi.org/10.1038/nature05403
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature05403
This article is cited by
-
Comparative morphology of sensilla of antennae, maxillary and labial palpi of adult Rhyzopertha dominica (F.) (Coleoptera: Bostrichidae), with specific reference to the typology and possible functions
The Journal of Basic and Applied Zoology (2023)
-
Adult oviposition preference and larval performance of Cnaphalocrocis medinalis Guenee (Pyralidae: Lepidoptera) on transgenic Bt rice
International Journal of Tropical Insect Science (2023)
-
Isolate-Specific Effect of Entomopathogenic Endophytic Fungi on Population Growth of Two-Spotted Spider Mite (Tetranychus urticae Koch) and Levels of Steroidal Glycoalkaloids in Tomato
Journal of Chemical Ecology (2021)
-
Drosophila taste neurons as an agonist-screening platform for P2X receptors
Scientific Reports (2020)
-
Cellular and molecular mechanisms of DEET toxicity and disease-carrying insect vectors: a review
Genes & Genomics (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.