Abstract
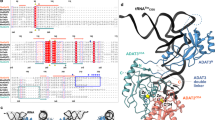
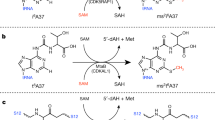
Uridine at the first anticodon position (U34) of glutamate, lysine and glutamine transfer RNAs is universally modified by thiouridylase into 2-thiouridine (s2U34), which is crucial for precise translation by restricting codon–anticodon wobble during protein synthesis on the ribosome. However, it remains unclear how the enzyme incorporates reactive sulphur into the correct position of the uridine base. Here we present the crystal structures of the MnmA thiouridylase–tRNA complex in three discrete forms, which provide snapshots of the sequential chemical reactions during RNA sulphuration. On enzyme activation, an α-helix overhanging the active site is restructured into an idiosyncratic β-hairpin-containing loop, which packs the flipped-out U34 deeply into the catalytic pocket and triggers the activation of the catalytic cysteine residues. The adenylated RNA intermediate is trapped. Thus, the active closed-conformation of the complex ensures accurate sulphur incorporation into the activated uridine carbon by forming a catalytic chamber to prevent solvent from accessing the catalytic site. The structures of the complex with glutamate tRNA further reveal how MnmA specifically recognizes its three different tRNA substrates. These findings provide the structural basis for a general mechanism whereby an enzyme incorporates a reactive atom at a precise position in a biological molecule.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Björk, G. R. Biosynthesis and function of modified nucleosides. In tRNA: Structure, Biosynthesis and Function (eds Söll, D. & RajBhandary, U. L.) 165–205 (American Society for Microbiology, Washington DC, 1995)
Yokoyama, S. & Nishimura, S. Modified nucleosides and codon recognition. In tRNA: Structure, Biosynthesis, and Function (eds Söll, D. & RajBhandary, U. L.) 207–224 (American Society for Microbiology, Washington DC, 1995)
Yokoyama, S. et al. Molecular mechanism of codon recognition by tRNA species with modified uridine in the first position of the anticodon. Proc. Natl Acad. Sci. USA 82, 4905–4909 (1985)
Ashraf, S. S. et al. Single atom modification (O → S) of tRNA confers ribosome binding. RNA 5, 188–194 (1999)
Krüger, M. K., Pedersen, S., Hagervall, T. G. & Sørensen, M. A. The modification of the wobble base of tRNAGlu modulates the translation rate of glutamic acid codons in vivo. J. Mol. Biol. 284, 621–631 (1998)
Urbonavicius, J., Qian, Q., Durand, J. M., Hagervall, T. G. & Björk, G. R. Improvement of reading frame maintenance is a common function for several tRNA modifications. EMBO J. 20, 4863–4873 (2001)
Sylvers, L. A., Rogers, K. C., Shimizu, M., Ohtsuka, E. & Söll, D. A 2-thiouridine derivative in tRNAGlu is a positive determinant for aminoacylation by Escherichia coli glutamyl-tRNA synthetase. Biochemistry 32, 3836–3841 (1993)
Isel, C., Marquet, R., Keith, G., Ehresmann, C. & Ehresmann, B. Modified nucleotides of tRNA3Lys modulate primer/template loop–loop interaction in the initiation complex of HIV-1 reverse transcription. J. Biol. Chem. 268, 25269–25272 (1993)
Isel, C. et al. Structural basis for the specificity of the initiation of HIV-1 reverse transcription. EMBO J. 18, 1038–1048 (1999)
Sullivan, M. A., Cannon, J. F., Webb, F. H. & Bock, R. M. Antisuppressor mutation in Escherichia coli defective in biosynthesis of 5-methylaminomethyl-2-thiouridine. J. Bacteriol. 161, 368–376 (1985)
Kambampati, R. & Lauhon, C. T. MnmA and IscS are required for in vitro 2-thiouridine biosynthesis in Escherichia coli. Biochemistry 42, 1109–1117 (2003)
Ikeuchi, Y., Shigi, N., Kato, J., Nishimura, A. & Suzuki, T. Mechanistic insights into sulfur relay by multiple sulfur mediators involved in thiouridine biosynthesis at tRNA wobble positions. Mol. Cell 21, 97–108 (2006)
Numata, T. et al. Crystallization and preliminary X-ray analysis of the tRNA thiolation enzyme MnmA from Escherichia coli complexed with tRNAGlu. Acta Crystallogr. F 62, 368–371 (2006)
Tesmer, J. J., Klem, T. J., Deras, M. L., Davisson, V. J. & Smith, J. L. The crystal structure of GMP synthetase reveals a novel catalytic triad and is a structural paradigm for two enzyme families. Nature Struct. Biol. 3, 74–86 (1996)
Rizzi, M. et al. Crystal structure of NH3-dependent NAD+ synthetase from Bacillus subtilis. EMBO J. 15, 5125–5134 (1996)
Nissen, P. et al. Crystal structure of the ternary complex of Phe-tRNAPhe, EF-Tu, and a GTP analog. Science 270, 1464–1472 (1995)
Palenchar, P. M., Buck, C. J., Cheng, H., Larson, T. J. & Mueller, E. G. Evidence that ThiI, an enzyme shared between thiamin and 4-thiouridine biosynthesis, may be a sulfurtransferase that proceeds through a persulfide intermediate. J. Biol. Chem. 275, 8283–8286 (2000)
Mueller, E. G., Palenchar, P. M. & Buck, C. J. The role of the cysteine residues of ThiI in the generation of 4-thiouridine in tRNA. J. Biol. Chem. 276, 33588–33595 (2001)
Weeks, C. M. & Miller, R. The design and implementation of SnB version 2.0. J. Appl. Cryst. 32, 120–124 (1999)
de La Fortelle, E. & Bricogne, G. Maximum-likelihood heavy-atom parameter refinement for multiple isomorphous replacement and multiwavelength anomalous diffraction methods. Methods Enzymol. 276, 472–494 (1997)
Abrahams, J. P. & Leslie, A. G. W. Methods used in the structure determination of bovine mitochondrial F1 ATPase. Acta Crystallogr. D 52, 30–42 (1996)
Jones, T. A., Zou, J. Y., Cowan, S. W. & Kjeldgaard, M. Improved methods for building protein models in electron density maps and the location of errors in these models. Acta Crystallogr. A 47, 110–119 (1991)
Brunger, A. T. et al. Crystallography & NMR system: A new software suite for macromolecular structure determination. Acta Crystallogr. D 54, 905–921 (1998)
Collaborative Computational Project Number 4. The CCP4 suite: Programs for protein crystallography. Acta Crystallogr. D 50, 760–763 (1994)
Laskowski, R. A., MacArthur, M. W., Moss, D. S. & Thornton, J. M. PROCHECK: A program to check the stereochemical quality of protein structures. J. Appl. Cryst. 26, 283–291 (1993)
Delano, W. L. The PyMOL molecular graphics system. v.0.97 http://www.pymol.org (2002).
Ishitani, R. CueMol: Molecular visualization framework. v.1.1 http://cuemol.sourceforge.jp (2006).
Ikeuchi, Y. et al. Molecular mechanism of lysidine synthesis that determines tRNA identity and codon recognition. Mol. Cell 19, 235–246 (2005)
Shigi, N., Suzuki, T., Tamakoshi, M., Oshima, T. & Watanabe, K. Conserved bases in the TPsi C loop of tRNA are determinants for thermophile-specific 2-thiouridylation at position 54. J. Biol. Chem. 277, 39128–39135 (2002)
Shigi, N. et al. Temperature-dependent biosynthesis of 2-thioribothymidine of Thermus thermophilus tRNA. J. Biol. Chem. 281, 2104–2113 (2006)
Acknowledgements
We thank M. Ibba for helpful improvement of the manuscript. We thank M. Kawamoto and N. Shimizu for their help in data collection at SPring-8. This work was supported by a PRESTO program grant from Japan Science and Technology (JST) to O.N., a grant for the National Project on Protein Structural and Functional Analyses from the Ministry of Education, Culture, Sports, Science and Technology (MEXT) to O.N., grants from MEXT to O.N., S.F. and T.S., a JSPS Fellowship for Japanese Junior Scientists to Y.I., the Asahi Glass Foundation to S.F., and Sumitomo and Kurata Memorial Hitachi Science and Technology Foundation grants to O.N. Author Contributions T.N. carried out structural determination and mutant preparation, and wrote the paper with editing from S.F. and O.N. S.F. and O.N. assisted the structural determination. Y.I. carried out the biochemical analyses under supervision of T.S. All authors discussed the results and commented on the manuscript. O.N. supervised all of the work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Coordinates and structure factors are deposited in the Protein Data Bank under accession code 2DER, 2DET and 2DEU for the initial tRNA-binding form, the prereaction form, and the adenylated intermediate form, respectively. Reprints and permissions information is available at npg.nature.com/reprintsandpermissions. The authors declare no competing financial interests.
Supplementary information
Supplementary Figures
This file contains Supplementary Figures 1–6 (PDF 60690 kb)
Supplementary Table 1
This table shows the data collection and refinement statistics. (PDF 86 kb)
Supplementary Notes
This file contains Supplementary Discussions, Supplementary Methods, Supplementary Figure Legends and additional references. (DOC 222 kb)
Rights and permissions
About this article
Cite this article
Numata, T., Ikeuchi, Y., Fukai, S. et al. Snapshots of tRNA sulphuration via an adenylated intermediate. Nature 442, 419–424 (2006). https://doi.org/10.1038/nature04896
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nature04896
This article is cited by
-
Sulphur shuttling across a chaperone during molybdenum cofactor maturation
Nature Communications (2015)
-
Structural basis for hypermodification of the wobble uridine in tRNA by bifunctional enzyme MnmC
BMC Structural Biology (2013)
-
Biogenesis of 2-agmatinylcytidine catalyzed by the dual protein and RNA kinase TiaS
Nature Structural & Molecular Biology (2011)
-
Structural basis of tRNA agmatinylation essential for AUA codon decoding
Nature Structural & Molecular Biology (2011)
-
Bacterial cysteine desulfurases: versatile key players in biosynthetic pathways of sulfur-containing biofactors
Applied Microbiology and Biotechnology (2011)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.