Abstract
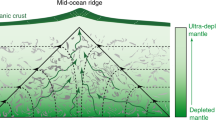
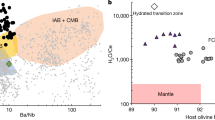
High 3He/4He ratios found in ocean island basalts are the main evidence for the existence of an undegassed mantle reservoir1,2,3. However, models of helium isotope evolution depend critically on the chemical behaviour of helium during mantle melting. It is generally assumed that helium is strongly enriched in mantle melts relative to uranium and thorium, yet estimates of helium partitioning in mantle minerals have produced conflicting results4,5,6. Here we present experimental measurements of helium solubility in olivine at atmospheric pressure. Natural and synthetic olivines were equilibrated with a 50% helium atmosphere and analysed by crushing in vacuo followed by melting, and yield a minimum olivine–melt partition coefficient of 0.0025 ± 0.0005 (s.d.) and a maximum of 0.0060 ± 0.0007 (s.d.). The results indicate that helium might be more compatible than uranium and thorium during mantle melting and that high 3He/4He ratios can be preserved in depleted residues of melting. A depleted source for high 3He/4He ocean island basalts would resolve the apparent discrepancy7 in the relative helium concentrations of ocean island and mid-ocean-ridge basalts.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Kurz, M. D., Jenkins, W. J. & Hart, S. R. Helium isotopic systematics of oceanic islands and mantle heterogeneity. Nature 297, 43–47 (1982)
Moreira, M., Doucet, S., Madureira, P. M., Lecomte, A. & Allègre, C. J. Helium-neon systematics in OIB and the nature of the source of mantle plumes. Geochim. Cosmochim. Acta 68, A283 (2004)
Allegre, C. J., Staudacher, T., Sarda, P. & Kurz, M. Constraints on the evolution of the Earth's mantle from rare-gas systematics. Nature 303, 762–766 (1983)
Hiyagon, H. & Ozima, M. Partition of noble-gases between olivine and basalt melt. Geochim. Cosmochim. Acta 50, 2045–2057 (1986)
Brooker, R. A., Heber, V., Kelley, S. P. & Wood, B. J. Noble gas partitioning behaviour during mantle melting: a possible explanation for ‘the He paradox’? EOS 84, Abstract V31F–03 (2003)
Marty, B. & Lussiez, P. Constraints on rare-gas partition-coefficients from analysis of olivine glass from a picritic midocean ridge basalt. Chem. Geol. 106, 1–7 (1993)
Anderson, D. L. The helium paradoxes. Proc. Natl Acad. Sci. USA 95, 4822–4827 (1998)
Graham, D. W. in Noble Gases in Geochemistry and Cosmochemistry (eds Porcelli, D., Ballentine, C. J. & Wieler, R.) 247–317 (The Mineralogical Society of America, Washington DC, 2002)
Kurz, M. D. Mantle heterogeneity beneath oceanic islands—some inferences from isotopes. Phil. Trans. R. Soc. Lond. A 342, 91–103 (1993)
Meibom, A. et al. Are high He-3/He-4 ratios in oceanic basalts an indicator of deep-mantle plume components? Earth Planet. Sci. Lett. 208, 197–204 (2003)
Yamamato, J. & Burnard, P. G. Solubility controlled noble gas fractionation during magmatic degassing: implications for noble gas compositions of primary melts of OIB and MORB. Geochim. Cosmochim. Acta 69, 727–734 (2005)
Honda, M. & Patterson, D. B. Systematic elemental fractionation of mantle-derived helium, neon, and argon in mid-oceanic ridge glasses. Geochim. Cosmochim. Acta 63, 2863–2874 (1999)
Workman, R. K. et al. Recycled metasomatized lithosphere as the origin of the enriched mantle II (EM2) end-member: evidence from the Samoan volcanic chain. Geochem. Geophys. Geosyst. 5, doi:10.1029/2003GC000623 (2004)
Burnard, P. G., Graham, D. W. & Farley, K. A. Mechanisms of magmatic gas loss along the Southeast Indian Ridge and the Amsterdam–St. Paul Plateau. Earth Planet. Sci. Lett. 203, 131–148 (2002)
Broadhurst, C. L., Drake, M. J., Hagee, B. E. & Bernatowicz, T. J. Solubility and Partitioning of Ne, Ar, Kr, and Xe in minerals and synthetic basaltic melts. Geochim. Cosmochim. Acta 56, 709–723 (1992)
Lux, G. The behaviour of noble-gases in silicate liquids—solution, diffusion, bubbles and surface effects, with applications to natural samples. Geochim. Cosmochim. Acta 51, 1549–1560 (1987)
Jambon, A., Weber, H. & Braun, O. Solubility of He, Ne, Ar, Kr and Xe in a basalt melt in the range 1250–1600 degrees C. Geochim. Cosmochim. Acta 50, 401–408 (1986)
Kurz, M. D., Curtice, J., Lott, D. E. & Solow, A. Rapid helium isotopic variability in Mauna Kea shield lavas from the Hawaiian Scientific Drilling Project. Geochem. Geophys. Geosyst. 5, doi:10.1029/2002GC000439 (2004)
Yokochi, R., Marty, B., Pik, R. & Burnard, P. High 3He/4He ratios in peridotite xenoliths from SW Japan revisited: Evidence for cosmogenic 3He released by vacuum crushing. Geochem. Geophys. Geosyst. 6, doi:10.1029/2004GC000836 (2005)
Nakamura, A. & Schmalzried, H. On the nonstoichiometry and point-defects of olivine. Phys. Chem. Miner. 10, 27–37 (1983)
Brooker, R. A. et al. The ‘zero charge’ partitioning behaviour of noble gases during mantle melting. Nature 423, 738–741 (2003)
Baker, M. B. & Stolper, E. M. Determining the composition of high-pressure mantle melts using diamond aggregates. Geochim. Cosmochim. Acta 58, 2811–2827 (1994)
Dick, H. J. B., Fisher, R. L. & Bryan, W. B. Mineralogic variability of the uppermost mantle along mid-ocean ridges. Earth Planet. Sci. Lett. 69, 88–106 (1984)
Gill, J. B. Orogenic Andesites and Plate Tectonics (Springer, New York, 1981)
Boyet, M. & Carlson, R. W. 142Nd evidence for early (> 4.53 Ga) global differentiation of the Silicate Earth. Science (in the press) (2005)
Class, C. & Goldstein, S. L. Evolution of helium isotopes in the Earth's mantle. Nature 436, 1107–1112 (2005)
Trull, T. W. & Kurz, M. D. Experimental measurements of He-3 and He-4 mobility in olivine and clinopyroxene at magmatic temperatures. Geochim. Cosmochim. Acta 57, 1313–1324 (1993)
Dunai, T. J. & Baur, H. Helium, neon, and argon systematics of the European subcontinental mantle—implications for its geochemical evolution. Geochim. Cosmochim. Acta 59, 2767–2783 (1995)
Landwehr, D., Blundy, J., Chamorro-Perez, E. M., Hill, E. & Wood, B. U-series disequilibria generated by partial melting of spinel lherzolite. Earth Planet. Sci. Lett. 188, 329–348 (2001)
Stuart, F. M., Lass-Evans, S., Fitton, J. G. & Ellam, R. M. High 3He/4He ratios in picritic basalts from Baffin Island and the role of a mixed reservoir in mantle plumes. Nature 424, 57–59 (2003)
Acknowledgements
We thank J. Curtice for his long labours with the He analyses, and J. van Orman for assistance in the early planning and execution of the project. This research was supported by the NSF. Author Contributions S.W.P. and T.L.G. performed the experiments and microscopic observations. M.D.K. performed the He analysis. S.R.H. contributed to experimental and analytical design. All authors contributed to data analysis.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Reprints and permissions information is available at npg.nature.com/reprintsandpermissions. The authors declare no competing financial interests.
Supplementary information
Supplementary Table 1
This table contains the run conditions of the experiments reported in the paper. (DOC 30 kb)
Supplementary Table 2
This table contains the analyses of the experiments. (DOC 88 kb)
Supplementary Table 3
This table contains the references used to calculate the average partition coefficients shown in Figure 4. (DOC 23 kb)
Rights and permissions
About this article
Cite this article
Parman, S., Kurz, M., Hart, S. et al. Helium solubility in olivine and implications for high 3He/4He in ocean island basalts. Nature 437, 1140–1143 (2005). https://doi.org/10.1038/nature04215
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nature04215
This article is cited by
-
A helium stratified and ingassed lower mantle: resolving the helium paradoxes
Acta Geochimica (2020)
-
Do Supercontinent-Superplume Cycles Control the Growth and Evolution of Continental Crust?
Journal of Earth Science (2020)
-
Chemical structure of the Earth’s mantle defined by fast diffusion elements like helium
Acta Geochimica (2020)
-
Hadean silicate differentiation preserved by anomalous 142Nd/144Nd ratios in the Réunion hotspot source
Nature (2018)
-
The structure and composition of olivine grain boundaries: 40 years of studies, status and current developments
Physics and Chemistry of Minerals (2018)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.