Abstract
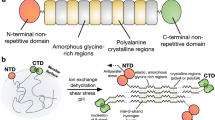
Resilin is a member of a family of elastic proteins that includes elastin, as well as gluten, gliadin, abductin and spider silks. Resilin is found in specialized regions of the cuticle of most insects, providing low stiffness, high strain and efficient energy storage1,2; it is best known for its roles in insect flight3,4 and the remarkable jumping ability of fleas5,6 and spittle bugs7. Previously, the Drosophila melanogaster CG15920 gene was tentatively identified as one encoding a resilin-like protein8,9 (pro-resilin). Here we report the cloning and expression of the first exon of the Drosophila CG15920 gene as a soluble protein in Escherichia coli. We show that this recombinant protein can be cast into a rubber-like biomaterial by rapid photochemical crosslinking. This observation validates the role of the putative elastic repeat motif in resilin function. The resilience (recovery after deformation) of crosslinked recombinant resilin was found to exceed that of unfilled synthetic polybutadiene, a high resilience rubber. We believe that our work will greatly facilitate structural investigations into the functional properties of resilin and shed light on more general aspects of the structure of elastomeric proteins. In addition, the ability to rapidly cast samples of this biomaterial may enable its use in situ for both industrial and biomedical applications.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Andersen, S. O. The crosslinks in resilin identified as dityrosine and trityrosine. Biochim. Biophys. Acta 93, 213–215 (1964)
Gosline, J. et al. Elastic proteins: biological roles and mechanical properties. Phil. Trans. R. Soc. Lond. B 357, 121–132 (2002)
Weis-Fogh, T. A rubber-like protein in insect cuticle. J. Exp. Biol. 37, 887–907 (1960)
Gorb, S. N. Serial elastic elements in the damselfly wing: mobile vein joints contain resilin. Naturwissenschaften 86, 552–555 (1999)
Neville, A. C. & Rothschild, M. Fleas—insects which fly with their legs. Proc. R. Entomol. Soc. Lond. 32, 9–10 (1967)
Rothschild, M. & Schlein, J. The jumping mechanism of Xenopsylla cheopis. I. Exoskeletal structures and musculature. Phil. Trans. R. Soc. Lond. B 271, 457–490 (1975)
Burrows, M. Biomechanics: froghopper insects leap to new heights. Nature 424, 509 (2003)
Ardell, D. H. & Andersen, S. O. Tentative identification of a resilin gene in Drosophila melanogaster. Insect Biochem. Mol. Biol. 31, 965–970 (2001)
Andersen, S. O. in Elastomeric Proteins (eds Shewry, P. R., Tatham, A. S. & Bailey, A. J.) 259–278 (Cambridge Univ. Press, Cambridge, UK, 2002)
Weis-Fogh, T. Molecular interpretation of the elasticity of resilin, a rubber-like protein. J. Mol. Biol. 3, 520–531 (1961)
Weis-Fogh, T. in The Cell and the Organism (eds Ramsay, J. A. & Wigglesworth, V. B.) 283–300 (Cambridge Univ. Press, Cambridge, UK, 1961)
Anderson, S. O. Covalent cross-links in a structural protein, resilin. Acta Physiol. Scand. Suppl. 263, 1–81 (1966)
Li, B. & Daggett, V. Molecular basis for the extensibility of elastin. J. Muscle Res. Cell Motil. 23, 561–573 (2002)
Rousseau, R., Schreiner, E., Kohlmeyer, A. & Marx, D. Temperature-dependent conformational transitions and hydrogen-bond dynamics of the elastin-like octapeptide GVG(VPGVG): A molecular-dynamics study. Biophys. J. 86, 1393–1407 (2004)
Pometun, M. S., Chekmenev, E. Y. & Wittebort, R. J. Quantitative observation of backbone disorder in native elastin. J. Biol. Chem. 279, 7982–7987 (2004)
Young, D. & Bennet-Clark, H. C. The role of the tymbal in cicada sound production. J. Exp. Biol. 198, 1001–1019 (1995)
Skals, N. & Surlykke, A. Sound production by abdominal tymbal organs in two moth species: The green silver-line and the scarce silver-line (Noctuoidea: Nolidae: Chloephorinae). J. Exp. Biol. 202, 2937–2949 (1999)
Andersen, S. O. & Weis-Fogh, T. Resilin: a rubber-like protein in arthropod cuticle. Adv. Insect Physiol. 2, 1–65 (1964)
Varman, A. R. Resilin in the cuticle of physogastric queen termites. Experientia 36, 564 (1980)
Singaravelu, G. Occurrence of elastic protein resilin in the spermatophore walls of a tick Haemaphysalis intermedia. Natl Acad. Sci. Lett. (India) 14, 147–149 (1991)
Tatham, A. S. & Shewry, P. R. Comparative structures and properties of elastic proteins. Phil. Trans. R. Soc. Lond. B 357, 229–234 (2002)
Malencik, D. A. & Anderson, S. R. Dityrosine formation in calmodulin: cross-linking and polymerization catalyzed by Arthromyces peroxidase. Biochemistry 35, 4375–4386 (1996)
Fancy, D. A. & Kodadek, T. Chemistry for the analysis of protein–protein interactions: rapid and efficient cross-linking triggered by long wavelength light. Proc. Natl Acad. Sci. USA 96, 6020–6024 (1999)
Urry, D. W. et al. in Elastomeric Proteins (eds Shewry, P. R., Tatham, A. S. & Bailey, A. J.) 54–93 (Cambridge Univ. Press, Cambridge, UK, 2002)
Keeley, F. W., Bellingham, C. M. & Woodhouse, K. A. Elastin as a self-organising biomaterial: use of recombinantly expressed human elastin polypeptides as a model system for investigations of structure and self-assembly of elastin. Phil. Trans. R. Soc. Lond. B 357, 185–189 (2002)
Neff, D., Frazier, S. F., Quimby, L., Wang, R. T. & Zill, S. Identification of resilin in the leg of cockroach, Periplaneta americana: confirmation by a simple method using pH dependence of UV fluorescence. Arthropod Struct. Dev. 29, 75–83 (2000)
Cowie, J. M. G. Polymers: Chemistry and Physics of Modern Materials (Int. Textbook Co. Ltd, Aylesbury, 1973)
Aaron, B. B. & Gosline, J. M. Elastin as a random-network elastomer: a mechanical and optical analysis of single elastin fibers. Biopolymers 20, 1247–1260 (1980)
Treloar, L. R. G. The Physics of Rubber Elasticity (Clarendon, Oxford, 1975)
Lehmann, F. O. & Dickinson, M. H. The production of elevated flight force compromises manoeuvrability in the fruit fly Drosophila melanogaster. J. Exp. Biol. 204, 627–635 (2001)
Acknowledgements
We thank J. Abbenante for preparative scale reverse-phase HPLC purification of dityrosine and for mass spectrometry. The technical assistance of L. Conlan is acknowledged for HPLC analysis of dityrosine. We are grateful to A. Brownlee and R. Tellam for critical reading of the manuscript. This work was supported by a CSIRO Nanotechnology Emerging Sciences Initiative grant.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Reprints and permissions information is available at npg.nature.com/reprintsandpermissions. The authors declare no competing financial interests.
Supplementary information
Suppplementary Notes
This file contains the Supplementary Methods and accompanying Supplementary Figures. (DOC 5736 kb)
Rights and permissions
About this article
Cite this article
Elvin, C., Carr, A., Huson, M. et al. Synthesis and properties of crosslinked recombinant pro-resilin. Nature 437, 999–1002 (2005). https://doi.org/10.1038/nature04085
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nature04085
This article is cited by
-
Programmable adhesion and morphing of protein hydrogels for underwater robots
Nature Communications (2024)
-
Cartilage-like protein hydrogels engineered via entanglement
Nature (2023)
-
Barrier properties of Nup98 FG phases ruled by FG motif identity and inter-FG spacer length
Nature Communications (2023)
-
Droplet superpropulsion in an energetically constrained insect
Nature Communications (2023)
-
Quick photofabrication of functional nanospheres from de novo designed peptides for NIR fluorescence and MR imaging
Nano Research (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.