Abstract
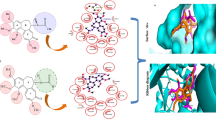
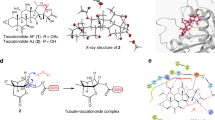
Vinblastine is one of several tubulin-targeting Vinca alkaloids that have been responsible for many chemotherapeutic successes since their introduction in the clinic as antitumour drugs1. In contrast with the two other classes of small tubulin-binding molecules (Taxol2 and colchicine3), the binding site of vinblastine is largely unknown and the molecular mechanism of this drug has remained elusive. Here we report the X-ray structure of vinblastine bound to tubulin in a complex with the RB3 protein stathmin-like domain (RB3-SLD). Vinblastine introduces a wedge at the interface of two tubulin molecules and thus interferes with tubulin assembly. Together with electron microscopical and biochemical data, the structure explains vinblastine-induced tubulin self-association into spiral aggregates at the expense of microtubule growth4. It also shows that vinblastine and the amino-terminal part of RB3-SLD binding sites share a hydrophobic groove on the α-tubulin surface that is located at an intermolecular contact in microtubules. This is an attractive target for drugs designed to perturb microtubule dynamics by interfacial interference, for which tubulin seems ideally suited because of its propensity to self-associate.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Jordan, M. A. & Wilson, L. Microtubules as a target for anticancer drugs. Nature Rev. Cancer 4, 253–265 (2004)
Nogales, E., Wolf, S. G. & Downing, K. H. Structure of the αβ tubulin dimer by electron crystallography. Nature 391, 199–203 (1998)
Ravelli, R. B. G. et al. Insight into tubulin regulation from a complex with colchicine and a stathmin-like domain. Nature 428, 198–202 (2004)
Himes, R. H. Interactions of the catharanthus (Vinca) alkaloids with tubulin and microtubules. Pharmacol. Ther. 51, 257–267 (1991)
Trouet, A. B., Hannart, J. A. A. & Rao, K. S. B. (Omnichem S.A., Belg), US Patent 4,639,456 (1987).
Ennifar, E., Carpentier, P., Ferrer, J. L., Walter, P. & Dumas, P. X-ray-induced debromination of nucleic acids at the Br K absorption edge and implications for MAD phasing. Acta Crystallogr. D 58, 1262–1268 (2002)
Ravelli, R. B. G., Leiros, H. K., Pan, B., Caffrey, M. & McSweeney, S. Specific radiation damage can be used to solve macromolecular crystal structures. Structure 11, 217–224 (2003)
Nogales, E., Whittaker, M., Milligan, R. A. & Downing, K. H. High-resolution model of the microtubule. Cell 96, 79–88 (1999)
Nogales, E., Downing, K. H., Amos, L. A. & Löwe, J. Tubulin and FtsZ form a distinct family of GTPases. Nature Struct. Biol. 5, 451–458 (1998)
Rai, S. S. & Wolff, J. Localization of the vinblastine-binding site on β-tubulin. J. Biol. Chem. 271, 14707–14711 (1996)
Mitra, A. & Sept, D. Localization of the antimitotic peptide and depsipeptide binding site on beta-tubulin. Biochemistry 43, 13955–13962 (2004)
Usui, T. et al. The anticancer natural product pironetin selectively targets Lys352 of α-tubulin. Chem. Biol. 11, 799–806 (2004)
Lobert, S. et al. Vinca alkaloid-induced tubulin spiral formation correlates with cytotoxicity in the leukemic L1210 cell line. Biochemistry 39, 12053–12062 (2000)
Sackett, D. L. Vinca site agents induce structural changes in tubulin different from and antagonistic to changes induced by colchicine site agents. Biochemistry 34, 7010–7019 (1995)
Na, G. C. & Timasheff, S. N. Interaction of vinblastine with calf brain tubulin. Biochemistry 25, 6214–6222 (1986)
Weisenberg, R. C. & Timasheff, S. N. Aggregation of microtubule subunit protein. Effects of divalent cations, colchicine and vinblastine. Biochemistry 9, 4110–4116 (1970)
Haskins, K. M., Donoso, J. A. & Himes, R. H. Spirals and paracrystals induced by Vinca alkaloids: evidence that microtubule-associated proteins act as polycations. J. Cell Sci. 47, 237–247 (1981)
Gigant, B. et al. The 4 Å X-ray structure of a tubulin:stathmin-like domain complex. Cell 102, 809–816 (2000)
Müller-Reichert, T., Chrétien, D., Severin, F. & Hyman, A. A. Structural changes at microtubule ends accompanying GTP hydrolysis: Information from a slowly hydrolyzable analogue of GTP, guanylyl (α,β)methylenediphosphonate. Proc. Natl Acad. Sci. USA 95, 3661–3666 (1998)
Nogales, E., Wang, H. W. & Niederstrasser, H. Tubulin rings: which way do they curve? Curr. Opin. Struct. Biol. 13, 256–261 (2003)
Steinmetz, M. O. et al. Op18/stathmin caps a kinked protofilament-like tubulin tetramer. EMBO J. 19, 572–580 (2000)
Wilson, L., Jordan, M. A., Morse, A. & Margolis, R. L. Interaction of vinblastine with steady-state microtubules in vitro . J. Mol. Biol. 159, 125–149 (1982)
Toso, R. J., Jordan, M. A., Farrell, K. W., Matsumoto, B. & Wilson, L. Kinetic stabilization of microtubule dynamic instability in vitro by vinblastine. Biochemistry 32, 1285–1293 (1993)
Jordan, M. A., Thrower, D. & Wilson, L. Mechanism of inhibition of cell proliferation by Vinca alkaloids. Cancer Res. 51, 2212–2222 (1991)
Charbaut, E. et al. Stathmin family proteins display specific molecular and tubulin binding properties. J. Biol. Chem. 276, 16146–16154 (2001)
Redeker, V. et al. Probing the native structure of stathmin and its interaction domains with tubulin. J. Biol. Chem. 275, 6841–6849 (2000)
Ogawa, T., Nitta, R., Okada, Y. & Hirokawa, N. A common mechanism for microtubule destabilizers-M type kinesins stabilize curling of the protofilament using the class-specific neck and loops. Cell 116, 591–602 (2004)
Renault, L., Guibert, B. & Cherfils, J. Structural snapshots of the mechanism and inhibition of a guanine nucleotide exchange factor. Nature 426, 525–530 (2003)
Bau, R. & Jin, K. K. Crystal structure of vinblastine. J. Chem. Soc. Perkin Trans. 1, 2079–2082 (2000)
Schuttelkopf, A. W. & van Aalten, D. M. PRODRG: a tool for high-throughput crystallography of protein–ligand complexes. Acta Crystallogr. D 60, 1355–1363 (2004)
Acknowledgements
F.R. thanks F. Guéritte and D. Guénard for support. We thank M.-F. Carlier for help and discussions. We are grateful to the Interdisciplinary Center for Microscopy of the University of Basel for access to the transmission electron microscope. This work was supported by the Association pour la Recherche sur le Cancer, the Centre National pour la Recherche Scientifique and the Institut National pour la Santé et la Recherche Médicale. Diffraction data were collected on beamline ID14-4 at European Synchrotron Radiation Facility. C.W. was supported in part by the K.C. Wong foundation and by an ARC postdoctoral fellowship.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Coordinates and structure factors have been deposited in the Protein Data Bank under accession number 1Z2B (tubulin–colchicine:RB3-SLD–vinblastine). Reprints and permissions information is available at npg.nature.com/reprintsandpermissions. The authors declare no competing financial interests.
Supplementary information
Supplementary Figure S1
Electron micrograph of negatively stained vinblastine-induced tubulin-colchicine curls. (DOC 160 kb)
Supplementary Figure S2
Interference between vinblastine and the straight tubulin assembly in protofilaments. (DOC 166 kb)
Supplementary Figure S3
The change of M-loop position after vinblastine-binding to a straight protofilament. (DOC 394 kb)
Supplementary Figure Legends
Legends to accompany the above Supplementary Figures S1-S3. (DOC 22 kb)
Supplementary Methods
Details on the proteins and buffers, structure determination and illustrations used in the study. (DOC 20 kb)
Supplementary Table S1
Crystallographic data and refinement statistics. (DOC 23 kb)
Rights and permissions
About this article
Cite this article
Gigant, B., Wang, C., Ravelli, R. et al. Structural basis for the regulation of tubulin by vinblastine. Nature 435, 519–522 (2005). https://doi.org/10.1038/nature03566
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nature03566
This article is cited by
-
Folylpolyglutamate synthetase mRNA G-quadruplexes regulate its cell protrusion localization and enhance a cancer cell invasive phenotype upon folate repletion
BMC Biology (2023)
-
Advanced application of nanotechnology in active constituents of Traditional Chinese Medicines
Journal of Nanobiotechnology (2023)
-
Multi-responsive chitosan-based hydrogels for controlled release of vincristine
Communications Chemistry (2023)
-
Xanthatin and 8-epi-xanthatin as new potential colchicine binding site inhibitors: a computational study
Journal of Molecular Modeling (2023)
-
Fukuyama reduction, Fukuyama coupling and Fukuyama–Mitsunobu alkylation: recent developments and synthetic applications
Molecular Diversity (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.