Abstract
Expression of multiple oncogenes and inactivation of tumour suppressors is required to transform primary mammalian cells into cancer cells1,2,3. Activated Ha-RasV12 (Ras) is usually associated with cancer, but it also produces paradoxical premature senescence4 in primary cells by inducing reactive oxygen species5 followed by accumulation of tumour suppressors p53 and p16INK4a (ref. 4). Here we identify, using a direct genetic screen, Seladin-1 (also known as Dhcr24) as a key mediator of Ras-induced senescence. Following oncogenic and oxidative stress, Seladin-1 binds p53 amino terminus and displaces E3 ubiquitin ligase Mdm2 from p53, thus resulting in p53 accumulation. Additionally, Seladin-1 associates with Mdm2 independently of p53, potentially affecting other Mdm2 targets. Ablation of Seladin-1 causes the bypass of Ras-induced senescence in rodent and human fibroblasts, and allows Ras to transform these cells. Wild-type Seladin-1, but not mutants that disrupt its association with either p53 or Mdm2, suppresses the transformed phenotype. The same mutants are also inactive in directing p53-dependent oxidative stress response. These results show an unanticipated role for Seladin-1, previously implicated in Alzheimer's disease6 and cholesterol metabolism7, in integrating cellular response to oncogenic and oxidative stress.
Similar content being viewed by others
Main
We decided to search for regulators of oncogene-induced senescence using a targeted genetic screen (Fig. 1a) with a genetic suppressor element (GSE)8 complementary DNA library prepared from Rat1 cells. We expected that inactivation of genes essential for Ras-induced senescence by GSE sequences would allow cells to escape senescence, continue cell division and eventually form transformed cell colonies in the presence of ectopic expression of an activated Ras allele (Ha-RasV12). By using this screen, we isolated multiple independent antisense GSE sequences corresponding to a broadly expressed oxidoreductase gene (Fig. 1b, Supplementary Fig. 1), initially identified in plants (DIMINUTO/DWARF1)9 with the human orthologue originally described in neuronal cells (Seladin-1)6, and later shown to encode 3β-hydroxysterol-Δ24-reductase activity (DHCR24)7, further referred to here as Seladin-1.
a, A genetic screen for regulators of Ras-induced premature senescence. GSE, genetic suppressor element. b, Seladin-1 expression in adult mouse tissues (northern). GAPDH, loading control. kb, kilobases. sk., skeletal. c, Left: western blot of Seladin-1 protein in rat embryo fibroblasts (REFs) and REFs expressing Seladin-1 GSE (pBABEpuro-R1-4). Right: SA-β-gal staining of REFs infected with vector pBABEpuro or pBABEpuro-R1-4. d, SA-β-gal staining of REFs infected with pBABEpuro or pBABEpuro-R1-4 and transduced with pWZLhygro or pWZLhygro-Ras (upper row); tumorigenicity in nude mice (lower row). e, Left: western blot of Seladin-1 in naïve WI38-TERT cells (lane 1), three clones transduced with pBABEpuro-Seladin-1si (lanes 2–4) or pBABEpuro (lanes 5–7). Right: penetrance of the senescent phenotype (SA-β-gal assay). Values are the means+s.d. (n = 3). f, Upper row: SA-β-gal staining of WI38-TERT cells transduced with either empty pBABEpuro (left and right) or pBABEpuro-Seladin-1si (middle) following transduction with pWZLneo-RasV12 (middle and right) or with pWZLneo vector (left). Lower row: anchorage-independent growth in soft agar of U2OS cells (left), WI38-TERT cells transduced with pBABEpuro-Seladin-1si plus pWZLneo-HaRasV12 (middle) and pBABEpuro-Seladin-1si alone (right).
To confirm the role of Seladin-1 in senescence-inducing Ras signalling, early passage rat embryo fibroblasts (REFs) were infected with retrovirus expressing Seladin-1 GSE (pBABEpuroR1-4; Supplementary Fig. 1). This resulted in a significant reduction of Seladin-1 messenger RNA (not shown) and protein levels (up to 90%; Fig. 1c, left panel), with no apparent effect on cell growth or morphology (Fig. 1c, right panel). Upon introduction of Ras through retroviral infection, REF cells co-infected with the control vector (pBABEpuro) were arrested in 6–10 days, as expected4. The ‘flat’ cellular morphology and positive staining of these cells in senescence-associated β-galactosidase (SA-β-gal) assay10 indicated that they were senescent (Fig. 1d, upper left). In contrast, upon Ras introduction, REF cells with Seladin-1 knockdown continued to proliferate, exhibited a ‘condensed’ cell morphology, and were not stained in SA-β-gal assay (Fig. 1d, upper right panel). Furthermore, these cells formed tumours within two weeks when inoculated into nude mice (5/5 cases), indicating that they were oncogenically transformed (Fig. 1d, bottom right).
As rodent and human cells behave differently with regards to oncogenic transformation4,11, we investigated whether Seladin-1 would have a similar effect on Ras-induced senescence in human cells. We used stable RNAi (RNA interference)12 to silence Seladin-1 expression in human WI38 fibroblasts that were immortalized by the ectopic telomerase (TERT) expression. Western blot analysis indicated that Seladin-1 protein expression was abrogated in WI38-TERT cells by Seladin-1 siRNA (small interfering RNA, pBABEpuro-Seladin-1si; Fig. 1e, and Supplementary Methods). When Seladin-1-ablated WI38-TERT cells were transduced with Ras, similar phenotype was observed as in Seladin-1 knockdown REFs (Fig. 1e, f and Supplementary Results), indicating that Seladin-1 is involved in regulating Ras-induced transformation and senescence both in human and rodent cells.
Ras-induced senescence is typically associated with activation of tumour suppressors p53, p16INK4a and, in mouse cells, p19ARF (refs 4, 13). Intriguingly, while Ras-mediated p16INK4a induction was unaffected in Seladin-1-ablated WI38-TERT cells, silencing of Seladin-1 prevented Ras-induced p53 accumulation (Fig. 2a). p14ARF shows only slight response to oncogenic Ras, which is in line with previous observations14,15. This suggests that Seladin-1 is an essential mediator of Ras-induced p53 response. Premature senescence initiated by Ras depends on the increase of intracellular reactive oxygen species (ROS)5. Accordingly, scavengers of hydrogen peroxide (H2O2), a potent ROS producer, rescue Ras-induced senescence. Moreover, sublethal doses of H2O2 provoke a senescence-like phenotype in human fibroblasts16.
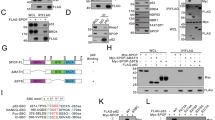
a,b, Western blot (WB) analysis; a, WI38-TERT/pBABEpuro and WI38-TERT/pBABEpuro-Seladin-1si cells with (+) or without (- ) ectopic Ha-RasV12 (Ras) transduction by WZLneoRasV12 retrovirus; b, Expression analysis of p53 and Seladin-1 in whole cell extracts (lanes 1 and 2) and Seladin-1 immunoprecipitates (IP; lanes 3–5) in the presence (+) (lanes 2, 4, 5) or absence (- ) (lanes 1, 3) of H2O2 treatment. Lane 5, cells treated with N-acetylcysteine (NAC). c, Effect of Seladin-1 mutants on p53 binding in PA-1 cells. Immunoprecipitates from cells expressing empty pcDNA3 (Vector), wild-type Seladin-1 (WT), mutants N294T/K306N and S419N before (- ) and after (+) treatment with H2O2, then blotted with p53 antibody. Anti-Flag shows the recombinant Seladin-1 variants expression. d, p53 and p21 in WI38-TERT and WI38-TERT expressing pBABEpuro-Seladin-1si. H2O2 treatment (upper panel), UV irradiation (middle panel), or γ-irradiation (lower panel). e, Western blot analysis of p53 levels in naïve WI38-TERT cells or WI38-TERT ectopically expressing Ha-RasV12, in the presence/absence of H2O2 and NAC as indicated. f, Top panel; anti-p53 western blot after IP with anti-Mdm2 antibodies of extracts from WI38-TERT cells treated with H2O2. Other panels; direct western blot of the same cell extracts with anti-p53, p21, Mdm2 and GAPDH antibodies. g–i, Seladin-1/Mdm2 IP-western blot of WI38-TERT (g), WI38-TERT and U2OS (h), and MEF and p53-/- MEF cells (i) following H2O2 treatment as indicated. Asterisk in h indicates the 60-kDa Mdm2 fragment expressed in U2OS cells. Lower panel in i corresponds to same Seladin-1 IP re-blotted with anti-Seladin-1 antibodies. j, Immunoblot with anti-Seladin-1 antibodies of anti-Flag IPs (top panel) or direct western blot with anti-Flag antibodies of whole cell extracts obtained following PA-1 cell transfection with the appropriate Flag-tagged p53 mutants. k, Homology between the Mdm2 binding region of p53 and the P-box of Seladin-1, and the p53 binding region of Mdm2 and the M-box of Seladin-1. Alignment was obtained using ClustalW27 (http://www.ebi.ac.uk/clustalw/#). Up arrowheads, p53 or Seladin-1 mutations inactivating both p53–Seladin-1 and p53–Mdm2 interaction (for p53 mutants) or Seladin-1–Mdm2 interaction (for Seladin-1 mutants). Δ, Deletion of the M-box from Seladin-1. Down arrowhead, S419N Seladin-1 mutant.
On these grounds, we sought to investigate whether Seladin-1 is integrated in Ras-initiated signalling downstream of ROS. Sublethal doses of H2O2 rapidly induced p53 and its downstream target, p21Cip1/Waf1, in control cells (Fig. 2d, top panel). In contrast, H2O2 treatment did not cause a significant change in p53 and p21Cip1/Waf1 protein levels in Seladin-1 knockdown cells, indicating that Seladin-1 is required for p53-mediated cellular response to ROS.
Moreover, we found that Seladin-1 is upregulated in response to both oxidative stress and Ras activation. Seladin-1 was induced in a time-dependent manner in WI38-TERT cells following H2O2 treatment, reaching the maximum level in 10–12 h (Fig. 3c). Similarly, Seladin-1 levels increased in response to Ras expression (Fig. 3d). Intriguingly, we found that Seladin-1 became associated with p53 in response to both H2O2 treatment (Fig. 2b) and Ras activation (Supplementary Fig. 2). In this context, the hydrogen peroxide scavenger N-acetylcysteine (NAC) prevented accumulation of p53 (ref. 5 and Fig. 2e, top panel) and abrogated Seladin-1–p53 interaction (Fig. 2b). This suggests that increases in intracellular ROS trigger p53 and Seladin-1 interaction.
a,b, Immunofluorescence staining of: a, Seladin-1 in WI38-TERT cells (left column), H2O2-treated cells (10 h after treatment; middle column) and pWZLneo-Ras-infected cells (10 days after infection; right column). Seladin-1 was stained with rhodamine (red), and nuclei counterstained with DAPI (blue). b, Seladin-1 in WI38-TERT cells transduced with Ras. Confocal microscopy on progressive cell sections showing Seladin-1 (Alexa 488, green), DNA (propidium iodide, red) and co-localization, yellow. c–e, Western blot analysis of: c, Seladin-1 in WI38-TERT cells before and after H2O2 treatment. d, Seladin-1 in WI38-TERT cells transduced with pWZLneo (lane 1), pWZLneo-Ras alone (lane 2) or together with NAC treatment (lane 3), naïve WI38-TERT cells (lane 4), WI38-TERT cells treated with H2O2 (lane 5) and with H2O2 and NAC together (lane 6) as in Fig. 2c. e, Seladin-1 in WI38-TERT cells before (0 h) and after γ-irradiation (0.5–48 h). f, Immunofluorescence staining of Seladin-1 in WI38-TERT cells before and 12 h after γ-irradiation (upper), and in WI38-TERT/pWZLneo-Ras cells without and with NAC treatment (lower). Scale bars, 10 µm. GAPDH, loading control.
Immunolocalization studies indicated that Seladin-1, normally confined to the perinuclear cytoplasmic region (ref. 6 and Fig. 3a, left column), concentrated in the nuclei after oxidative challenge (Fig. 3a, middle column and Supplementary Fig. 3). Similar changes in Seladin-1 subcellular localization were also observed in WI38-TERT cells expressing a constitutively active Ras allele (Fig. 3a, right column and 3b). Treatment of these cells with NAC inhibited Seladin-1 induction (Fig. 3d) and its nuclear translocation (Fig. 3f, bottom panel), suggesting that Ras-promoted translocation or nuclear retention of Seladin-1 was mediated by ROS signalling.
Seladin-1 levels and localization were mostly unaffected by mild doses of UV or ionizing radiation (IR; here γ-irradiation), indicating that the cellular response to these stresses does not seem to require Seladin-1 (Fig. 3e and f). Accordingly, Seladin-1 ablation failed to prevent UV- and IR-induced p53-dependent growth arrest (Figs 2d, 4e). Taken together, these results support a role of Seladin-1 specifically in the p53 response to oncogenic and oxidative stress which might be determined by the nature of damage caused by different stresses17,18.
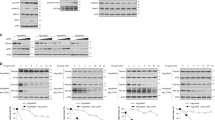
a, Effect of Seladin-1 on REF transformation by p53(R175H) and Ha-RasV12. Normalized numbers of transformed foci for vector, wild-type Seladin-1 (Sel-1), and mutant Seladin-1 (Sel-1(S419N); Sel-1(N294T/K306N); Sel-1(L210Q/F211S) and Sel-1(ΔM) (as in Fig. 2k)) are shown. The values were normalized to the number of foci observed on the control vector plates. The columns in the graph represent normalized average + s.d. (n = 3). b, Effect of Seladin-1 mutations on its interaction with p53. Top panel, IP with anti-Flag, followed by immunoblot with anti-p53. Bottom panel, Flag immunoblot. P-box Seladin-1 mutant still binds p53. c, Inhibition of p53–Mdm2 interaction by the recombinant Seladin-1. Top row, immunoblot of Flag-p53-bound Mdm2 following incubation with increasing amounts of GST-Seladin-1. Second row, p53-associated Seladin-1 from the same experiment detected with anti-Seladin-1 antibody. Bottom two rows, direct immunoblot of total Flag-p53 or Mdm2 at the conclusion of the experiment. d, Inhibition of Mdm2 ubiquitination of p53 in vitro by Seladin-1. Asterisk, unspecific band on top of p53. e, Effect of H2O2 treatment on DNA replication (BrdU incorporation, upper graph) and on senescence induction (SA-β-gal staining, lower graph) in WI38-TERT cells and cells expressing Seladin-1 siRNA (Seladin-1si). Results are mean percentages + s.d. (n = 3). f, Model of Ras- and oxidative stress-initiated signalling pathway. H, human; M, mouse.
To gain further insight into the mechanism of the Seladin-1 effect on p53, we prepared p53 mutants and tested their interaction with Seladin-1 in co-immunoprecipitation (IP) assays. Interestingly, N-terminal p53 mutant(s), deficient in p53–Mdm2 interaction, also lost the ability to bind Seladin-1, suggesting that Seladin-1 associates with p53 at the Mdm2 binding site of the latter (Fig. 2j). In contrast, mutants of the p53 DNA binding domain retain interaction with Seladin-1 (Fig. 2j). Surprisingly, we also observed an association between Seladin-1 and Mdm2 (Fig. 2g–i) that was more pronounced in extracts from cells subjected to oxidative stress (Fig. 2g–i). Moreover, Seladin-1–Mdm2 interaction was independent of p53 and p14ARF, since it was observed also in p53 null (MEF p53 -/ - ) and p14ARF null cells (U2OS) under similar conditions (Fig. 2h, i). These results suggest that Seladin-1 might affect p53 by interfering with Mdm2-mediated p53 regulation.
To test this hypothesis, we prepared bacterially expressed Flag-p53, Seladin-1 and Mdm2 (Supplementary Fig. 7) and found that, first, Seladin-1 binds p53 and displaces Mdm2 from p53 in vitro (Fig. 4c). Second, recombinant Seladin-1 inhibits in vitro ubiquitination of Flag-p53 by Mdm2 (Fig. 4d). Finally, levels of Mdm2-associated p53 decrease during oxidative stress in vivo (Fig. 2f, top panel) despite concomitant increase in overall p53 and stable Mdm2 levels in the same experiments (Fig. 2f).
Sequence analysis revealed that Seladin-1 contains two adjacent regions of homology with the Mdm2-binding site on p53 (Box P) and with the p53-binding site on Mdm2 (Box M; Fig. 2k and Supplementary Fig. 5). The significance of these sites for Seladin-1–p53 and Seladin-1–Mdm2 interactions is underlined by failure of the appropriate Seladin-1 mutants to interact with p53 (Figs 2c, 4b) and Mdm2 (Supplementary Fig. 6), respectively. Additionally, the Seladin-1 N294T/K306N double mutation, which inactivates its intrinsic DHCR24 activity7, has no effect on Seladin-1–p53 interaction (Fig. 2c). Our data support a model where Seladin-1 directly inhibits Mdm2–p53 interaction following oxidative stress, causing a decrease in Mdm2-dependent p53 ubiquitination and degradation, and resulting in accumulation of active p53 and subsequent initiation of the cell cycle arrest. Thus, Seladin-1 shows features of a potential tumour suppressor involved in cellular response to Ras/p53-mediated oncogenic signalling and oxidative stress.
Hence, Seladin-1 suppresses oncogenic foci formation promoted by co-expression of dominant negative p53 and oncogenic Ras in REFs (Fig. 4a). This activity depends on p53 and Mdm2 binding, since both M-box and P-box Seladin-1 mutants were ineffective (Fig. 4a, b; Fig. 2c and Supplementary Fig. 6). In contrast, abrogation of DHCR24 activity (N294T/K306N double mutant) had little effect on growth suppression (Fig. 4a) and p53 accumulation following oxidative challenge (Supplementary Fig. 4).
Inactivation of either p53 or p19ARF in combination with an activated Ras allele suffices to transform rodent primary cells. In contrast, p53 mutation fails to overcome Ras-induced senescence in human primary cells4,19. We observed that Seladin-1 ablation uniquely prevents human fibroblasts from arresting in response to Ras, while maintaining Ras growth stimulatory potential. This suggests that in addition to p53, Seladin-1 might affect other pathways relevant to Ras-mediated premature senescence in human cells. Accordingly, since Seladin-1 also interacts with Mdm2 independently of p53, this suggests a possible effect on other Mdm2 targets, such as pRB20,21,22,23,24 (Fig. 4f). Additionally, since Seladin-1 displaces Mdm2 from p53, other p53 modifications catalysed by Mdm225 might also be affected by Seladin-1.
The similarity of the Seladin-1 response to Ras and oxidative stress suggests that Seladin-1 may unexpectedly serve as an important ROS effector in mammalian cells. Concordantly, we observed that cells with Seladin-1 knockdown continued to show BrdU incorporation (Fig. 4e, top panel), cell proliferation (Supplementary Fig. 8) and approximately fourfold less SA-β-gal staining (Fig. 4e, lower panel) after H2O2 treatment, suggesting an essential role for Seladin-1 in the oxidative stress response senescence program16. Interestingly, Ras activation and ROS generation were not affected by Seladin-1 (Supplementary Fig. 9), placing Seladin-1 downstream of Ras and ROS in premature senescence and oxidative stress response pathways (Fig. 4f).
Seladin-1 downregulation coincides with Alzheimer's disease6, but the molecular basis of this correlation remains unclear. The only biological activity of Seladin-1 described previously was 3β-hydroxysterol-Δ24-reductase (DHCR24) that catalyses the reduction of desmosterol, the last step in cholesterol biosynthesis7. We show here that the DHCR24 activity of Seladin-1 is apparently not required for p53 binding and p53-dependent oxidative stress response (Fig. 2c and Supplemental Fig. 4). Nevertheless, two seemingly independent Seladin-1 functions affecting cholesterol and p53 might share some regulatory mechanisms. In fact, oxidation of cholesterol produces ROS26 and this gives rise to a potential crosstalk between these two pathways, with Seladin-1 acting as a potential sensor and/or effector of oxidative stress.
Ras and tumour suppressors p53 and p14ARF/p19ARF are involved in oncogenesis, cellular senescence and ageing. Additionally, both oxidative stress and cholesterol metabolism have been implicated in ageing and degenerative disorders at the organismal level. Our data support the notion that Seladin-1 stands at the intersection of all these processes.
Methods
Vectors and cell culture
Retroviral vectors pBABEpuro, pWZLhygro and pWZLneo were used to construct pBABEpuro-Seladin-1, pBABEpuro-R1-4 and pBABEpuro-Seladin-1si, pWZLhygro-H-rasV12 and pWZLneo-H-rasV12. pWZLhygro-hTERT was a gift from R. Weinberg. Retrovirus preparation, infection and selection were done as described4. Cell lines and growth conditions are described in Supplementary Methods.
GSE cDNA library screen
The retroviral GSE screen was performed as previously described8; details are given in Supplementary Methods. Antisense GSE sequences recovered from the screen were used to search against available NCBI databases to find the corresponding genes and their homologues in different species. Accession numbers in GenBank for the corresponding genes are: human Seladin-1/DHCR24 cDNA D13463 (partial), AF261758 (full); genomic clone AC009946; mouse cDNA AY039762; Arabidopsis thaliana DWF1 U12400; Caenorhabditis elegans homologue AF026214.
Senescence-associated β-galactosidase staining
The staining was performed essentially as described4. Cells were washed briefly with distilled water after staining and then examined under an inverted Olympus IX70 microscope.
Immunoprecipitation, western blot, cell growth and tumorigenesis
Cells were treated as described in Supplementary Methods.
siRNA inhibition of Seladin-1 expression
Two oligonucleotides were synthesized and annealed: 5′-GATCCCCTGGAAGGAGCAGGGTAGCATTCAAGAGATGCTACCCTGCTCCTTCCATTTTTGGAAA-3′ and 5′-AGCTTTTCCAAAAATGGAAGGAGCAGGGTAGCATCTCTTGAATGCTACCCTGCTCCTTCCAGGG-3′ (siRNA target recognition sequence is underlined). The annealed double-strand oligonucleotide was then inserted into the BglII-HindIII sites of pSUPER12. To improve the RNAi efficiency, the siRNA expression cassette (BamHI-SalI fragment) was removed from pSUPER-Seladin-1si and cloned into BamHI-SalI digested pBABEpuro. The resultant pBABEpuro-Seladin-1si was used to generate retrovirus and infect WI38-TERT cells. Puromycin selection was applied for three days to generate cells with a stable inhibition of Seladin-1.
Seladin-1, mutants, expression, purification, ubiquitination assays
Detailed procedures are described in Supplementary Methods.
BrdU incorporation assay
The assay was done using the in situ Cell Proliferation Kit, FLUOS, following the instructions (Roche). Cells were cultured on coverslips, and BrdU labelling was for 4 h. The BrdU signal was examined under an inverted Olympus IX70 microscope equipped with proper fluorescence filters.
References
Ruley, H. E. Adenovirus early region 1A enables viral and cellular transforming genes to transform primary cells in culture. Nature 304, 602–606 (1983)
Land, H., Parada, L. F. & Weinberg, R. A. Tumorigenic conversion of primary embryo fibroblasts requires at least two cooperating oncogenes. Nature 304, 596–602 (1983)
Hahn, W. C. et al. Creation of human tumour cells with defined genetic elements. Nature 400, 464–468 (1999)
Serrano, M., Lin, A. W., McCurrach, M. E., Beach, D. & Lowe, S. W. Oncogenic ras provokes premature cell senescence associated with accumulation of p53 and p16INK4a. Cell 88, 593–602 (1997)
Lee, A. C. et al. Ras proteins induce senescence by altering the intracellular levels of reactive oxygen species. J. Biol. Chem. 274, 7936–7940 (1999)
Greeve, I. et al. The human DIMINUTO/DWARF1 homolog seladin-1 confers resistance to Alzheimer's disease-associated neurodegeneration and oxidative stress. J. Neurosci. 20, 7345–7352 (2000)
Waterham, H. R. et al. Mutations in the 3β-hydroxysterol Δ24-reductase gene cause desmosterolosis, an autosomal recessive disorder of cholesterol biosynthesis. Am. J. Hum. Genet. 69, 685–694 (2001)
Gudkov, A. V. et al. Cloning mammalian genes by expression selection of genetic suppressor elements: association of kinesin with drug resistance and cell immortalization. Proc. Natl Acad. Sci. USA 91, 3744–3748 (1994)
Takahashi, T., Gasch, A., Nishizawa, N. & Chua, N. H. The DIMINUTO gene of Arabidopsis is involved in regulating cell elongation. Genes Dev. 9, 97–107 (1995)
Dimri, G. P. et al. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc. Natl Acad. Sci. USA 92, 9363–9367 (1995)
Hahn, W. C. & Weinberg, R. A. Modelling the molecular circuitry of cancer. Nature Rev. Cancer 2, 331–341 (2002)
Brummelkamp, T. R., Bernards, R. & Agami, R. A system for stable expression of short interfering RNAs in mammalian cells. Science 296, 550–553 (2002)
Lin, A. W. & Lowe, S. W. Oncogenic ras activates the ARF-p53 pathway to suppress epithelial cell transformation. Proc. Natl Acad. Sci. USA 98, 5025–5030 (2001)
Brookes, S. et al. INK4a-deficient human diploid fibroblasts are resistant to RAS-induced senescence. EMBO J. 21, 2936–2945 (2002)
Dimri, G. P., Itahana, K., Acosta, M. & Campisi, J. Regulation of a senescence checkpoint response by the E2F1 transcription factor and p14(ARF) tumor suppressor. Mol. Cell Biol. 20, 273–285 (2000)
Chen, Q. M. et al. Molecular analysis of H2O2-induced senescent-like growth arrest in normal human fibroblasts: p53 and Rb control G1 arrest but not cell replication. Biochem. J. 332, 43–50 (1998)
Cadet, J., Berger, M., Douki, T. & Ravanat, J. L. Oxidative damage to DNA: formation, measurement, and biological significance. Rev. Physiol. Biochem. Pharmacol. 131, 1–87 (1997)
Hoeijmakers, J. H. Genome maintenance mechanisms for preventing cancer. Nature 411, 366–374 (2001)
Wei, W., Hemmer, R. M. & Sedivy, J. M. Role of p14(ARF) in replicative and induced senescence of human fibroblasts. Mol. Cell. Biol. 21, 6748–6757 (2001)
Xiao, Z. et al. Interaction between the retinoblastoma protein and the oncoprotein MDM2. Nature 375, 694–698 (1995)
Sun, P., Dong, P., Dai, K., Hannon, G. J. & Beach, D. p53-independent role of MDM2 in TGF-β1 resistance. Science 282, 2270–2272 (1998)
Martin, K. et al. Stimulation of E2F1/DP1 transcriptional activity by MDM2 oncoprotein. Nature 375, 691–694 (1995)
Hsieh, J. et al. RB regulates the stability and the apoptotic function of p53 via MDM2. Mol. Cell 3, 181–193 (1999)
Ganguli, G. & Wasylyk, B. p53-independent functions of MDM2. Mol. Cancer Res. 1, 1027–1035 (2003)
Xirodimas, D., Saville, M. K., Bourdon, J. C., Hay, R. T. & Lane, D. P. Mdm2-mediated NEDD8 conjugation of p53 inhibits its transcriptional activity. Cell 118, 83–97 (2004)
Cutler, R. G. et al. Involvement of oxidative stress-induced abnormalities in ceramide and cholesterol metabolism in brain aging and Alzheimer's disease. Proc. Natl Acad. Sci. USA 101, 2070–2075 (2004)
Thompson, J. D., Higgins, D. G. & Gibson, T. J. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22, 4673–4680 (1994)
Acknowledgements
We thank R. Weinberg for pWZLhygro-TERT, R. Bernards for pSUPER, and D. Shalloway and J. L. Bos for GST-RBD. A. Osborn and M. Tetzlaff are thanked for help with Seladin-1 cDNA colony hybridization and early construct preparations. M. Serrano is thanked for INK4a/ARF knockout and control MEFs. We also thank L. Donehower for discussions and A. Beaudet for continuing support of this project. This work was supported in part by grants from US DOD and the Gustav and Louise Pfeiffer Research Foundation (K.G.) and AIRC (Italian Association for Cancer Research, R.M.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing financial interests.
Supplementary information
Supplementary Notes
Contains Supplementary Methods, Supplementary Data, Supplementary Figure Legends, Supplementary References. (DOC 67 kb)
Supplementary Figure S1
Illustration of human Seladin-1, cDNA, siRNA and relative position of rat GSE sequences. (JPG 26 kb)
Supplementary Figure S2
Co-immunoprecipitation of Seladin-1 and p53 in Ras transduced cells. (JPG 25 kb)
Supplementary Figure S3
Seladin-1 in cytoplasmic and nuclear fractions. (JPG 25 kb)
Supplementary Figure S4
Effect of Seladin-1 mutants on oxidative stress response. (JPG 34 kb)
Supplementary Figure S5
Alignment of human, mouse, rat and soil nematode C. elegans Seladin-1 orthologues. (PDF 36 kb)
Supplementary Figure S6
Interaction between Seladin-1 and Mdm2. (JPG 21 kb)
Supplementary Figure S7
Recombinant proteins. (JPG 36 kb)
Supplementary Figure S8
Long term growth effect of H2O2 treatment on WI38-TERT or Seladin-1siWi38-TERT cells. (JPG 44 kb)
Supplementary Figure S9
Seladin-1 and Ras activity (panels A,B,D) or intracellular ROS level (panel C). (JPG 50 kb)
Rights and permissions
About this article
Cite this article
Wu, C., Miloslavskaya, I., Demontis, S. et al. Regulation of cellular response to oncogenic and oxidative stress by Seladin-1. Nature 432, 640–645 (2004). https://doi.org/10.1038/nature03173
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nature03173
This article is cited by
-
High fat diet induces brain injury and neuronal apoptosis via down-regulating 3-β hydroxycholesterol 24 reductase (DHCR24)
Cell and Tissue Research (2023)
-
The role of DHCR24 in the pathogenesis of AD: re-cognition of the relationship between cholesterol and AD pathogenesis
Acta Neuropathologica Communications (2022)
-
Protein disulfide isomerase A3 might be involved in the regulation of 24-dehydrocholesterol reductase via vitamin D equilibrium in primary cortical neurons
In Vitro Cellular & Developmental Biology - Animal (2021)
-
Serum seladin-1 levels in diabetes mellitus and Alzheimer's disease patients
Acta Neurologica Belgica (2020)
-
Oxidative Stress and Lymphocyte Alterations in Chronic Relapsing Experimental Allergic Encephalomyelitis in the Rat Hippocampus and Protective Effects of an Ethanolamine Phosphate Salt
Molecular Neurobiology (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.