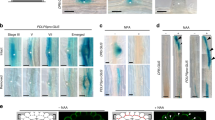
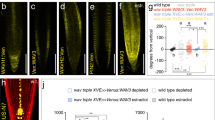
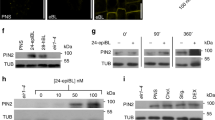
Abstract
Axis formation occurs in plants, as in animals, during early embryogenesis. However, the underlying mechanism is not known. Here we show that the first manifestation of the apical–basal axis in plants, the asymmetric division of the zygote, produces a basal cell that transports and an apical cell that responds to the signalling molecule auxin. This apical–basal auxin activity gradient triggers the specification of apical embryo structures and is actively maintained by a novel component of auxin efflux, PIN7, which is located apically in the basal cell. Later, the developmentally regulated reversal of PIN7 and onset of PIN1 polar localization reorganize the auxin gradient for specification of the basal root pole. An analysis of pin quadruple mutants identifies PIN-dependent transport as an essential part of the mechanism for embryo axis formation. Our results indicate how the establishment of cell polarity, polar auxin efflux and local auxin response result in apical–basal axis formation of the embryo, and thus determine the axiality of the adult plant.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
St Johnston, D. & Nüsslein-Volhard, C. The origin of pattern and polarity in the Drosophila embryo. Cell 68, 201–219 (1992)
Lyczak, R., Gomes, J. & Bowerman, B. Heads or tails: cell polarity and axis formation in the early Caenorhabditis elegans embryo. Dev. Cell 3, 157–166 (2002)
Jürgens, G. Apical–basal pattern formation in Arabidopsis embryogenesis. EMBO J. 20, 3609–3616 (2001)
Mayer, U., Torres Ruiz, R. A., Berleth, T., Miséra, S. & Jürgens, G. Mutations affecting body organization in the Arabidopsis embryo. Nature 353, 402–407 (1991)
Hamann, T., Mayer, U. & Jürgens, G. The auxin-insensitive bodenlos mutation affects primary root formation and apical–basal patterning in the Arabidopsis embryo. Development 126, 1387–1395 (1999)
Hardtke, C. & Berleth, T. The Arabidopsis gene MONOPTEROS encodes a transription factor mediating embryo axis formation and vascular development. EMBO J. 17, 1405–1411 (1998)
Hamann, T., Benkova, E., Bäurle, I., Kientz, M. & Jürgens, G. The Arabidopsis BODENLOS gene encodes an auxin response protein inhibiting MONOPTEROS-mediated embryo patterning. Genes Dev. 16, 1610–1615 (2002)
Steinmann, T. et al. Coordinated polar localization of auxin efflux carrier PIN1 by GNOM ARF-GEF. Science 286, 316–318 (1999)
Geldner, N. et al. The Arabidopsis GNOM ARF-GEF mediates endosomal recycling, auxin transport, and auxin-dependent plant growth. Cell 112, 219–230 (2003)
Liu, C., Xu, Z. & Chua, N. Auxin polar transport is essential for the establishment of bilateral symmetry during early plant embryogenesis. Plant Cell 5, 621–630 (1993)
Hadfi, K., Speth, V. & Neuhaus, G. Auxin-induced developmental patterns in Brassica juncea embryos. Development 125, 879–887 (1998)
Sabatini, S. et al. An auxin-dependent distal organizer of pattern and polarity in the Arabidopsis root. Cell 99, 463–472 (1999)
Friml, J. et al. AtPIN4 mediates sink-driven auxin gradients and root patterning in Arabidopsis. Cell 108, 661–673 (2000)
Fischer-Iglesias, C., Sundberg, B., Neuhaus, G. & Jones, A. Auxin distribution and transport during embryonic pattern formation in wheat. Plant J. 26, 115–129 (2001)
Ribnicky, D., Cohen, J., Hu, W. & Cooke, T. An auxin surge following fertilization in carrots: a mechanism for regulating plant totipotency. Planta 214, 505–509 (2002)
Friml, J. Auxin transport—shaping the plant. Curr. Opin. Plant Biol. 6, 7–12 (2003)
Rubery, P. & Sheldrake, A. Carrier-mediated auxin transport. Planta 118, 101–121 (1974)
Raven, J. Transport of indolacetic acid in plant cells in relation to pH and electrical potential gradients, and its significance for polar IAA transport. New Phytol. 74, 163–172 (1975)
Friml, J. & Palme, K. Polar auxin transport—old questions and new concepts? Plant Mol. Biol. 49, 273–284 (2002)
Okada, K., Ueda, J., Komaki, M., Bell, C. & Shimura, Y. Requirement of the auxin polar transport system in early stages of Arabidopsis floral bud formation. Plant Cell 3, 677–684 (1991)
Rashotte, A., Brady, S., Reed, R., Ante, S. & Muday, G. Basipetal auxin transport is required for gravitropism in roots of Arabidopsis. Plant Physiol. 122, 481–490 (2000)
Friml, J., Wisniewska, J., Benková, E., Mendgen, K. & Palme, K. Lateral relocation of auxin efflux regulator AtPIN3 mediates tropism in Arabidopsis. Nature 415, 806–809 (2002)
Ottenschläger, I. et al. Gravity-regulated differential auxin transport from columella to lateral root cap cells. Proc. Natl Acad. Sci. USA 100, 2987–2991 (2003)
Yamaizumi, M., Mekada, E., Uchida, T. & Okada, Y. One molecule of Diphteria Toxin fragment A introduced into a cell can kill the cell. Cell 15, 245–250 (1978)
Haseloff, J. GFP variants for multispectral imaging of living cells. Methods Cell Biol. 58, 139–151 (1999)
Weijers, D., Geldner, N., Offringa, R. & Jürgens, G. Early paternal gene activity in Arabidopsis. Nature 414, 709–710 (2001)
Weijers, D. Hormonal Regulation of Pattern Formation in the Arabidopsis Embryo. Thesis, Univ. Leiden (2002)
Caruso, J., Pence, V. & Leverone, L. in Plant Hormones: Physiology, Biochemistry and Molecular Biology (ed. Davies, P.) 43–447 (Kluwer Academic, The Netherlands, 1995)
Delbarre, A., Muller, P., Imhoff, V. & Guern, J. Comparison of mechanisms controlling uptake and accumulation of 2,4-dichlorophenoxy acetic acid, naphthalene-1-acetic acid, and indole-3-acetic acid in suspension-cultured tobacco cells. Planta 198, 532–541 (1996)
Sundaresan, V. et al. Patterns of gene action in plant development revealed by enhancer trap and gene trap transposable elements. Genes Dev. 14, 1797–1810 (1995)
Mayer, U., Büttner, G. & Jürgens, G. Apical–basal pattern formation in the Arabidopsis embryo: Studies on the role of the gnom gene. Development 117, 149–162 (1993)
Steeves, T. & Sussex, I. Patterns in Plant Development (Cambridge Univ. Press, Cambridge, 1989)
Teleman, A., Strigini, M. & Cohen, S. Shaping morphogen gradients. Cell 105, 559–562 (2001)
Tabata, T. Genetics of morphogen gradients. Nature Rev. Genet. 2, 620–630 (2001)
Ulmasov, T., Murfett, J., Hagen, G. & Guilfoyle, T. Aux/IAA proteins repress expression of reporter genes containing natural and highly active synthetic auxin response elements. Plant Cell 9, 1963–1971 (1997)
Braselton, J., Wilkinson, M. & Clulow, S. Feulgen staining of intact plant tissues for confocal microscopy. Biotechnol. Histochem. 71, 84–87 (1996)
Friml, J., Benkova, E., Mayer, U., Palme, K. & Muster, G. Automated whole mount localisation techniques for plant seedlings. Plant J. 34, 115–124 (2003)
Gälweiler, L. et al. Regulation of polar auxin transport by AtPIN1 in Arabidopsis vascular tissue. Science 282, 2226–2230 (1998)
Moctezuma, E. Changes in auxin patterns in developing gynophores of the peanut plant (Arachis hypogaea L.). Ann. Bot. 83, 235–242 (1999)
Acknowledgements
We thank S. Hiller, M. Kientz, G. Martin and M. L. O. Mendes for technical assistance, and C. Luschnig and K. Palme for providing material. Sequence-indexed Arabidopsis insertion mutants were obtained from the Salk Institute Genomic Analysis Laboratory and Cold Spring Harbor Laboratory. We are grateful to K. Cornelis and N. Geldner for critical reading of the manuscript. This work was supported by the Volkswagen Stiftung programme (M.S., J.F.), Landesgraduiertenförderung (A.V.), and the Research Council for Earth and Lifesciences (ALW), with financial aid from the Dutch Organization of Scientific Research (NWO) (D.W.) and the Deutsche Forschungsgemeinschaft.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing financial interests.
Supplementary information
Rights and permissions
About this article
Cite this article
Friml, J., Vieten, A., Sauer, M. et al. Efflux-dependent auxin gradients establish the apical–basal axis of Arabidopsis. Nature 426, 147–153 (2003). https://doi.org/10.1038/nature02085
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nature02085
This article is cited by
-
BRIP1 and BRIP2 maintain root meristem by affecting auxin-mediated regulation
Planta (2024)
-
NnWOX1-1, NnWOX4-3, and NnWOX5-1 of lotus (Nelumbo nucifera Gaertn)promote root formation and enhance stress tolerance in transgenic Arabidopsis thaliana
BMC Genomics (2023)
-
Antigravitropic PIN polarization maintains non-vertical growth in lateral roots
Nature Plants (2023)
-
Ab-GALFA, A bioassay for insect gall formation using the model plant Arabidopsis thaliana
Scientific Reports (2023)
-
Cis-regulatory elements and transcription factors related to auxin signaling in the streptophyte algae Klebsormidium nitens
Scientific Reports (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.