Abstract
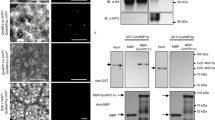
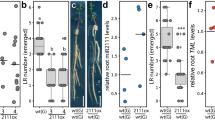
In legumes, root nodule organogenesis is activated in response to morphogenic lipochitin oligosaccharides that are synthesized by bacteria, commonly known as rhizobia1. Successful symbiotic interaction results in the formation of highly specialized organs called root nodules, which provide a unique environment for symbiotic nitrogen fixation. In wild-type plants the number of nodules is regulated by a signalling mechanism integrating environmental and developmental cues to arrest most rhizobial infections within the susceptible zone of the root2,3,4,5,6,7. Furthermore, a feedback mechanism controls the temporal and spatial susceptibility to infection of the root system. This mechanism is referred to as autoregulation of nodulation, as earlier nodulation events inhibit nodulation of younger root tissues3,4,8. Lotus japonicus plants homozygous for a mutation in the hypernodulation aberrant root (har1) locus escape this regulation and form an excessive number of nodules9,10,11. Here we report the molecular cloning and expression analysis of the HAR1 gene and the pea orthologue, Pisum sativum, SYM29. HAR1 encodes a putative serine/threonine receptor kinase, which is required for shoot-controlled regulation of root growth, nodule number, and for nitrate sensitivity of symbiotic development.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Spaink, H. P. Root nodulation and infection factors produced by rhizobial bacteria. Annu. Rev. Microbiol. 54, 257–288 (2000)
Nutman, P. S. Studies on the physiology of nodule formation. III. Experiments on the excision of root tip and nodules. Ann. Bot. 16, 81–102 (1952)
Pierce, M. & Bauer, W. D. A rapid regulatory response governing nodulation in soybean. Plant Physiol. 73, 286–290 (1983)
Kosslak, R. M. & Bohlool, B. B. Suppression of nodule development of one side of a split root system of soybeans caused by prior inoculation of the other side. Plant Physiol. 75, 125–130 (1984)
Carroll, B. J. & Mathews, A. Molecular Biology of Symbiotic Nitrogen Fixation (ed. Gresshoff, P.) 159–180 (CRS, Boca Raton, 1990)
Vasse, J., de Billy, F. & Truchet, G. Abortion of infection during the Rhizobium meliloti-alfalfa symbiotic interaction is accompanied by a hypersensitive reaction. Plant J. 4, 555–566 (1993)
Penmetsa, R. V. & Cook, D. R. A legume ethylene-insensitive mutant hyperinfected by its Rhizobial symbiont. Science 275, 527–530 (1997)
Caetano-Anolles, G. & Gresshoff, P. M. Plant genetic control of nodulation. Annu. Rev. Microbiol. 45, 345–382 (1991)
Schauser, L. et al. Symbiotic mutants deficient in nodule establishment identified after T-DNA transformation of Lotus japonicus. Mol. Gen. Genet. 259, 414–423 (1998)
Szczyglowski, K. et al. Nodule organogenesis and symbiotic mutants of the model legume Lotus japonicus. Mol. Plant-Microbe Interact. 11, 684–697 (1998)
Wopereis, J. et al. Short root mutant of Lotus japonicus with a dramatically altered symbiotic phenotype. Plant J. 23, 97–114 (2000)
Carroll, B. J., McNeil, D. L. & Gresshoff, P. M. Isolation and properties of soybean [Glycine max (L.) Merr.] mutants that nodulate in the presence of high nitrate concentrations. Proc. Natl Acad. Sci. USA 82, 4162–4166 (1985)
Delves, A. C. et al. Regulation of the soybean-Rhizobium nodule symbiosis by shoot and root factors. Plant Physiol. 82, 588–590 (1986)
Sheng, C. & Harper, J. E. Shoot versus root signal involvement in nodulation and vegetative growth in wild-type and hypernodulating soybean genotypes. Plant Physiol. 113, 825–831 (1997)
Sandal, N. et al. A genetic linkage map of the model legume Lotus japonicus and strategies for fast mapping of new loci. Genetics 161, 1673–1683 (2002)
Sato, S. et al. Structural analysis of the Lotus japonicus genome. I. Sequence features and mapping of fifty-six TAC clones which cover the 5.4 Mb regions of the genome. DNA Res. 8, 311–318 (2001)
Nakamura, Y. et al. Structural analysis of a Lotus japonicus genome. II. Sequence features and mapping of sixty-five TAC clones which cover the 6.5-Mb regions of the genome. DNA Res. 9, 63–70 (2002)
Clark, S. E., Williams, R. W. & Meyerowitz, E. M. The CLAVATA1 gene encodes a putative receptor kinase that controls shoot and floral meristem size in Arabidopsis. Cell 89, 575–585 (1997)
Shiu, S.-H. & Bleecker, A. B. Receptor-like kinases from Arabidopsis form a monophylitic gene family related to animal receptor kinases. Proc. Natl Acad. Sci. USA 11, 10763–10768 (2001)
Trotochaud, A. E., Hao, T., Wu, G., Yang, Z. & Clark, S. E. The CLAVATA1 receptor-like kinase requires CLAVATA3 for its assembly into a signalling complex that includes KAPP and Rho-related protein. Plant Cell 11, 393–405 (1999)
Yamamoto, E., Karakaya, H. C. & Knap, H. T. Molecular charcterization of two soybean homologs af Arabidopsis thaliana CLAVATA1 from the wild type and fasciation mutant. Biochem. Biophys. Acta 1491, 333–340 (2000)
Sagan, M. & Duc, G. Sym28 and Sym29, two new genes involved in regulation of nodulation in pea (Pisum sativum L.). Symbiosis 20, 229–245 (1996)
Torii, K. U. Receptor kinase activation and signal trunsduction in plants: an emerging picture. Curr. Opin. Plant Biol. 3, 361–367 (2000)
Clark, S. E. Cell signalling at the shoot meristem. Nature Rev. 2, 276–284 (2001)
Handberg, K. & Stougaard, J. Lotus japonicus, an autogamous diploid legume species for classical and molecular genetics. Plant J. 2, 487–496 (1992)
Kawaguchi, M. Lotus japonicus ‘Miyakojima’ MG20: An early-flowering accession suitable for indoor handling. J. Plant Res. 113, 507–509 (2000)
Vos, P. et al. AFLP: a new technique for DNA fingerprinting. Nucleic Acids Res. 23, 4407–4414 (1995)
Michelmore, R. W., Paran, I. & Kesseli, R. V. Identification of markers linked to disease-resistance genes by bulked segregant analysis. A rapid method to detect markers in specific genomic regions by using segregating populations. Proc. Natl Acad. Sci. USA 88, 9828–9832 (1991)
Hanks, S. K. & Quinn, A. M. Protein kinase catalytic domain sequence database: Identification of conserved features of primary structure and classification of family members. Methods Enzymol. 200, 38–62 (1988)
Acknowledgements
This work was funded by the Danish Ministry of Food, Agriculture and Fishery. Support was also provided by the National Science Foundation and the Kazusa DNA Research Institute Foundation. The Danish Agricultural and Veterinary Research Council supports L.K. We thank K. Keegstra for administrative support during the course of the work, A. Albin for administrative assistance, and H. de Larembergue for technical assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing financial interests.
Rights and permissions
About this article
Cite this article
Krusell, L., Madsen, L., Sato, S. et al. Shoot control of root development and nodulation is mediated by a receptor-like kinase. Nature 420, 422–426 (2002). https://doi.org/10.1038/nature01207
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature01207
This article is cited by
-
Innovations in functional genomics and molecular breeding of pea: exploring advances and opportunities
aBIOTECH (2024)
-
The developmental dynamics in cool season legumes with focus on chickpea
Plant Molecular Biology (2023)
-
Lotus japonicus regulates root nodulation and nitrogen fixation dependent on the molecular form of nitrogen fertilizer
Plant and Soil (2023)
-
Effective rhizobia enhance legume growth during subsequent drought despite water costs associated with nitrogen fixation
Plant and Soil (2023)
-
Lateral Root versus Nodule: The Auxin-Cytokinin Interplay
Journal of Plant Growth Regulation (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.