Abstract
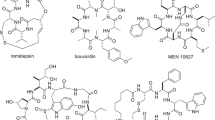
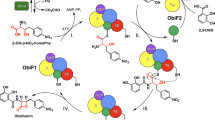
Molecules in nature are often brought to a bioactive conformation by ring formation (macrocyclization)1. A recurrent theme in the enzymatic synthesis of macrocyclic compounds by non-ribosomal and polyketide synthetases is the tethering of activated linear intermediates through thioester linkages to carrier proteins, in a natural analogy to solid-phase synthesis2. A terminal thioesterase domain of the synthetase catalyses release from the tether and cyclization3,4. Here we show that an isolated thioesterase can catalyse the cyclization of linear peptides immobilized on a solid-phase support modified with a biomimetic linker, offering the possibility of merging natural-product biosynthesis with combinatorial solid-phase chemistry. Starting from the cyclic decapeptide antibiotic tyrocidine A, this chemoenzymatic approach allows us to diversify the linear peptide both to probe the enzymology of the macrocyclizing enzyme, TycC thioesterase, and to create a library of cyclic peptide antibiotic products. We have used this method to reveal natural-product analogues of potential therapeutic utility; these compounds have an increased preference for bacterial over eukaryotic membranes and an improved spectrum of activity against some common bacterial pathogens.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Rizo, J. & Gierasch, L. M. Constrained peptides: models of bioactive peptides and protein substructures. Annu. Rev. Biochem. 61, 387–418 (1992)
Cane, D. E., Walsh, C. T. & Khosla, C. Harnessing the biosynthetic code: combinations, permutations, and mutations. Science 282, 63–68 (1998)
Marahiel, M. A., Stachelhaus, T. & Mootz, H. D. Modular peptide synthetases involved in nonribosomal peptide synthesis. Chem. Rev. 97, 2651–2674 (1997)
Keating, T. A. et al. Chain termination steps in nonribosomal peptide synthetase assembly lines: directed acyl-S-enzyme breakdown in antibiotic and siderophore biosynthesis. ChemBioChem 2, 99–107 (2001)
Lambalot, R. H. et al. A new enzyme superfamily — the phosphopantetheinyl transferases. Chem. Biol. 3, 923–936 (1996)
Trauger, J. W., Kohli, R. M., Mootz, H. D., Marahiel, M. A. & Walsh, C. T. Peptide cyclization catalyzed by the thioesterase domain of tyrocidine synthetase. Nature 407, 215–218 (2000)
Kohli, R. M., Trauger, J. W., Schwarzer, D., Marahiel, M. A. & Walsh, C. T. Generality of peptide cyclization catalyzed by isolated thioesterase domains of nonribosomal peptide synthetases. Biochemistry 40, 7099–7108 (2001)
Kuo, M. C. & Gibbons, W. A. Nuclear Overhauser effect and cross-relaxation rate determinations of dihedral and transannular interproton distances in the decapeptide tyrocidine A. Biophys. J. 32, 807–836 (1980)
Zasloff, M. Antimicrobial peptides of multicellular organisms. Nature 415, 389–395 (2002)
Nizet, V. et al. Innate antimicrobial peptide protects the skin from invasive bacterial infection. Nature 414, 454–457 (2001)
Li, J., Szittner, R., Derewenda, Z. S. & Meighen, E. A. Conversion of serine-114 to cysteine-114 and the role of the active site nucleophile in acyl transfer by myristoyl-ACP thioesterase from Vibrio harveyi. Biochemistry 35, 9967–9973 (1996)
Izumiya, N. Synthetic Aspects of Biologically Active Cyclic Peptides: Gramicidin S and Tyrocidines (Wiley, New York, 1979)
Hull, S. E., Karlsson, R., Main, P., Woolfson, M. M. & Dodson, E. J. The crystal structure of a hydrated gramicidin S-urea complex. Nature 275, 206–207 (1978)
Matsuzaki, K. Why and how are peptide-lipid interactions utilized for self-defense? Biochim. Biophys. Acta 1462, 1–10 (1999)
Hancock, R. E. & Lehrer, R. Cationic peptides: a new source of antibiotics. Trends Biotechnol. 16, 82–88 (1998)
Young, J. D., Leong, L. G., DiNome, M. A. & Cohn, Z. A. A semiautomated hemolysis microassay for membrane lytic proteins. Anal. Biochem. 154, 649–654 (1986)
Kondejewski, L. H. et al. Dissociation of antimicrobial and hemolytic activities in cyclic peptide diastereomers by systematic alterations in amphipathicity. J. Biol. Chem. 274, 13181–13192 (1999)
Scott, C. P., Abel-Santos, E., Jones, A. D. & Benkovic, S. J. Structural requirements for the biosynthesis of backbone cyclic peptide libraries. Chem. Biol. 8, 801–815 (2001)
Ovchinnikov, Y. A. & Ivanov, V. T. in The Proteins Vol. 5 (eds Neurath, H. & Hill, R. L.) 391–399 (Academic, New York, 1982)
Stachelhaus, T., Schneider, A. & Marahiel, M. A. Rational design of peptide antibiotics by targeted replacement of bacterial and fungal domains. Science 269, 69–72 (1995)
Acknowledgements
We thank R. Kolter for providing strains for antibacterial assays, and the Harvard Center for Proteomics for access to equipment. We also thank M. D. Burke and J.-M. Gauguet for discussions. This work was supported by the NIH (C.T.W.). R.M.K. is supported by the Medical Scientist Training Program, and M.D.B. was an NIH post-doctoral fellow.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing financial interests.
Rights and permissions
About this article
Cite this article
Kohli, R., Walsh, C. & Burkart, M. Biomimetic synthesis and optimization of cyclic peptide antibiotics. Nature 418, 658–661 (2002). https://doi.org/10.1038/nature00907
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nature00907
This article is cited by
-
A Novel Chemoenzymatic Approach to Produce Cilengitide Using the Thioesterase Domain from Microcystis aeruginosa Microcystin Synthetase C
The Protein Journal (2019)
-
Structural basis of nonribosomal peptide macrocyclization in fungi
Nature Chemical Biology (2016)
-
Discovery of MRSA active antibiotics using primary sequence from the human microbiome
Nature Chemical Biology (2016)
-
Enantiomeric discrimination of leucine enantiomers by nanotubular cyclic peptides: DFT and ONIOM calculation of the absorption spectra of guested enantiomers
Journal of Inclusion Phenomena and Macrocyclic Chemistry (2016)
-
The Emergence of Cyclic Peptides: The Potential of Bioengineered Peptide Drugs
International Journal of Peptide Research and Therapeutics (2014)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.