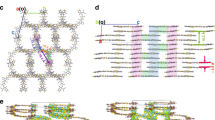
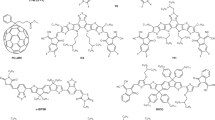
Abstract
Non-fullerene acceptors (NFAs) are currently a major focus of research in the development of bulk-heterojunction organic solar cells (OSCs). In contrast to the widely used fullerene acceptors (FAs), the optical properties and electronic energy levels of NFAs can be readily tuned. NFA-based OSCs can also achieve greater thermal stability and photochemical stability, as well as longer device lifetimes, than their FA-based counterparts. Historically, the performance of NFA OSCs has lagged behind that of fullerene devices. However, recent developments have led to a rapid increase in power conversion efficiencies for NFA OSCs, with values now exceeding 13%, demonstrating the viability of using NFAs to replace FAs in next-generation high-performance OSCs. This Review discusses the important work that has led to this remarkable progress, focusing on the two most promising NFA classes to date: rylene diimide-based materials and materials based on fused aromatic cores with strong electron-accepting end groups. The key structure–property relationships, donor–acceptor matching criteria and aspects of device physics are discussed. Finally, we consider the remaining challenges and promising future directions for the NFA OSCs field.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Cheng, Y., Yang, S. & Hsu, C. Synthesis of conjugated polymers for organic solar cell applications. Chem. Rev. 109, 5868–5923 (2009).
Li, G., Zhu, R. & Yang, Y. Polymer solar cells. Nat. Photonics 6, 153–161 (2012).
Krebs, F. C., Espinosa, N., Hosel, M., Sondergaard, R. R. & Jorgensen, M. 25th anniversary article: rise to power — OPV-based solar parks. Adv. Mater. 26, 29–38 (2014).
Darling, S. B. & You, F. The case for organic photovoltaics. RSC Adv. 3, 17633–17648 (2013).
Deibel, C. et al. Energetics of excited states in the conjugated polymer poly(3-hexylthiophene). Phys. Rev. B 81, 085202 (2010).
Yu, G., Gao, J., Hummelen, J. C., Wudl, F. & Heeger, A. J. Polymer photovoltaic cells - enhanced efficiencies via a network of internal donor-acceptor heterojunctions. Science 270, 1789–1791 (1995).
Vandewal, K., Tvingstedt, K., Gadisa, A., Inganas, O. & Manca, J. V. On the origin of the open-circuit voltage of polymer–fullerene solar cells. Nat. Mater. 8, 904–909 (2009).
Shockley, W. & Queisser, H. J. Detailed balance limit of efficiency of p-n junction solar cells. J. Appl. Phys. 32, 510–519 (1961).
Sworakowski, J., Lipinski, J. & Janus, K. On the reliability of determination of energies of HOMO and LUMO levels in organic semiconductors from electrochemical measurements. A simple picture based on the electrostatic model. Org. Electron. 33, 300–310 (2016).
Chen, H. et al. Polymer solar cells with enhanced open-circuit voltage and efficiency. Nat. Photonics 3, 649–653 (2009).
Liang, Y. et al. Development of new semiconducting polymers for high performance solar cells. J. Am. Chem. Soc. 131, 56–57 (2009).
Liang, Y. & Yu, L. A new class of semiconducting polymers for bulk heterojunction solar cells with exceptionally high performance. Acc. Chem. Res. 43, 1227–1236 (2010).
Li, Y. Molecular design of photovoltaic materials for polymer solar cells: toward suitable electronic energy levels and broad absorption. Acc. Chem. Res. 45, 723–733 (2012).
Chen, Y., Wan, X. & Long, G. High performance photovoltaic applications using solution-processed small molecules. Acc. Chem. Res. 46, 2645–2655 (2013).
Liao, S., Jhuo, H., Cheng, Y. & Chen, S. Fullerene derivative-doped zinc oxide nanofilm as the cathode of inverted polymer solar cells with low-bandgap polymer (PTB7-Th) for high performance. Adv. Mater. 25, 4766–4771 (2013).
Zhao, J. et al. Efficient organic solar cells processed from hydrocarbon solvents. Nat. Energy 1, 15027 (2016).
Li, M. et al. Solution-processed organic tandem solar cells with power conversion efficiencies >12%. Nat. Photonics 11, 85–90 (2017).
Distler, A. et al. The effect of PCBM dimerization on the performance of bulk heterojunction solar cells. Adv. Energy Mater. 4, 1300693 (2014).
Bloking, J. T. et al. Comparing the device physics and morphology of polymer solar cells employing fullerenes and non-fullerene acceptors. Adv. Energy Mater. 4, 1301426 (2013).
Jinnai, S. et al. Electron-accepting π-conjugated systems for organic photovoltaics: influence of structural modification on molecular orientation at donor–acceptor interfaces. Chem. Mater. 28, 1705–1713 (2016).
Su, G. et al. Linking morphology and performance of organic solar cells based on decacyclene triimide acceptors. J. Mater. Chem. A 2, 1781–1789 (2014).
Savoie, B. M. et al. Mesoscale molecular network formation in amorphous organic materials. Proc. Natl Acad. Sci. USA 111, 10055–10060 (2014).
Tang, C. W. Two-layer organic photovoltaic cell. Appl. Phys. Lett. 48, 183–185 (1986).
Halls, J. J. M. et al. Efficient photodiodes from interpenetrating polymer networks. Nature 376, 498–500 (1995).
Yu, G. & Heeger, A. J. Charge separation and photovoltaic conversion in polymer composites with internal donor/acceptor heterojunctions. J. Appl. Phys. 78, 4510–4515 (1995).
Anthony, J. E. Small-molecule, nonfullerene acceptors for polymer bulk heterojunction organic photovoltaics. Chem. Mater. 23, 583–590 (2011).
Sonar, P., Lim, J. P. F. & Chan, K. L. Organic non-fullerene acceptors for organic photovoltaics. Energy Environ. Sci. 4, 1558–1574 (2011).
Li, C. & Wonneberger, H. Perylene imides for organic photovoltaics: yesterday, today, and tomorrow. Adv. Mater. 24, 613–636 (2012).
Guo, X., Facchetti, A. & Marks, T. J. Imide- and amide-functionalized polymer semiconductors. Chem. Rev. 114, 8943–9021 (2014).
Lin, Y. & Zhan, X. Non-fullerene acceptors for organic photovoltaics: an emerging horizon. Mater. Horiz. 1, 470–488 (2014).
Lin, Y. & Zhan, X. Designing efficient non-fullerene acceptors by tailoring extended fused-rings with electron-deficient groups. Adv. Energy Mater. 5, 1501063 (2015).
Nielsen, C. B., Holliday, S., Chen, H., Cryer, S. J. & McCulloch, I. Non-fullerene electron acceptors for use in organic solar cells. Acc. Chem. Res. 48, 2803–2812 (2015).
Kang, H. et al. From fullerene-polymer to all-polymer solar cells: the importance of molecular packing, orientation, and morphology control. Acc. Chem. Res. 49, 2424–2434 (2016).
Lin, Y. & Zhan, X. Oligomer molecules for efficient organic photovoltaics. Acc. Chem. Res. 49, 175–183 (2016).
Zhao, W. et al. Molecular optimization enables over 13% efficiency in organic solar cells. J. Am. Chem. Soc. 139, 7148–7151 (2017).
Cui, Y. et al. Fine-tuned photoactive and interconnection layers for achieving over 13% efficiency in a fullerene-free tandem organic solar cell. J. Am. Chem. Soc. 139, 7302–7309 (2017).
Kumari, T., Lee, S. M., Kang, S. H., Chen, S. & Yang, C. Ternary solar cells with a mixed face-on and edge-on orientation enable an unprecedented efficiency of 12.1%. Energy Environ. Sci. 10, 258–265 (2017).
Kwon, O. K., Park, J.-H., Kim, D. W., Park, S. K. & Park, S. Y. An all-small-molecule organic solar cell with high efficiency nonfullerene acceptor. Adv. Mater. 27, 1951–1956 (2015).
Patil, Y., Misra, R., Keshtov, M. L. & Sharma, G. D. Small molecule carbazole-based diketopyrrolopyrroles with tetracyanobutadiene acceptor unit as a non-fullerene acceptor for bulk heterojunction organic solar cells. J. Mater. Chem. A 5, 3311–3319 (2017).
Shu, Y. et al. A survey of electron-deficient pentacenes as acceptors in polymer bulk heterojunction solar cells. Chem. Sci. 2, 363–368 (2011).
Li, H. et al. Beyond fullerenes: design of nonfullerene acceptors for efficient organic photovoltaics. J. Am. Chem. Soc. 136, 14589–14597 (2014).
Dang, M. et al. Bis(tri-n-alkylsilyl oxide) silicon phthalocyanines: a start to establishing a structure property relationship as both ternary additives and non-fullerene electron acceptors in bulk heterojunction organic photovoltaic devices. J. Mater. Chem. A 5, 12168–12182 (2017).
Long, X. et al. Polymer acceptor based on double B←N bridged bipyridine (BNBP) unit for high-efficiency all-polymer solar cells. Adv. Mater. 28, 6504–6508 (2016).
Cnops, K. et al. 8.4% Efficient fullerene-free organic solar cells exploiting long-range exciton energy transfer. Nat. Commun. 5, 3406 (2014).
Zhan, X. et al. Rylene and related diimides for organic electronics. Adv. Mater. 23, 268–284 (2011).
Suraru, S. L. & Wurthner, F. Strategies for the synthesis of functional naphthalene diimides. Angew. Chem. Int. Ed. 53, 7428–7448 (2014).
Pho, T. V., Toma, F. M., Chabinyc, M. L. & Wudl, F. Self-assembling decacyclene triimides prepared through a regioselective hextuple Friedel–Crafts carbamylation. Angew. Chem. Int. Ed. 52, 1446–1451 (2013).
Li, H. et al. Tetraazabenzodifluoranthene diimides: building blocks for solution-processable n-type organic semiconductors. Angew. Chem. Int. Ed. 52, 5513–5517 (2013).
Li, H. et al. Fine-tuning the 3D structure of nonfullerene electron acceptors toward high-performance polymer solar cells. Adv. Mater. 27, 3266–3272 (2015).
Shoaee, S. et al. Acceptor energy level control of charge photogeneration in organic donor/acceptor blends. J. Am. Chem. Soc. 132, 12919–12926 (2010).
Shoaee, S. et al. Charge photogeneration in polythiophene–perylene diimide blend films. Chem. Commun. 5445–5447 (2009).
Shin, W. S. et al. Effects of functional groups at perylene diimide derivatives on organic photovoltaic device application. J. Mater. Chem. 16, 384–390 (2006).
Schubert, A. et al. Ultrafast exciton self-trapping upon geometry deformation in perylene-based molecular aggregates. J. Phys. Chem. Lett. 4, 792–796 (2013).
Sharenko, A. et al. A high-performing solution-processed small molecule:perylene diimide bulk heterojunction solar cell. Adv. Mater. 25, 4403–4406 (2013).
Hartnett, P. E. et al. Slip-stacked perylenediimides as an alternative strategy for high efficiency nonfullerene acceptors in organic photovoltaics. J. Am. Chem. Soc. 136, 16345–16356 (2014).
Liu, J. et al. Fast charge separation in a non-fullerene organic solar cell with a small driving force. Nat. Energy 1, 16089 (2016).
Langhals, H. & Jona, W. Intense dyes through chromophore–chromophore interactions: bi- and trichromophoric perylene-3,4:9,10-bis(dicarboximide)s. Angew. Chem. Int. Ed. 37, 952–955 (1998).
Langhals, H. & Saulich, S. Bichromophoric perylene derivatives: energy transfer from non-fluorescent chromophores. Chem. Eur. J. 8, 5630–5643 (2002).
Holman, M. W., Yan, P., Adams, D. M., Westenhoff, S. & Silva, C. Ultrafast spectroscopy of the solvent dependence of electron transfer in a perylenebisimide dimer. J. Phys. Chem. A 109, 8548–8552 (2005).
Wilson, T. M., Tauber, M. J. & Wasielewski, M. R. Toward an n-type molecular wire: electron hopping within linearly linked perylenediimide oligomers. J. Am. Chem. Soc. 131, 8952–8957 (2009).
Rajaram, S., Shivanna, R., Kandappa, S. K. & Narayan, K. S. Nonplanar perylene diimides as potential alternatives to fullerenes in organic solar cells. J. Phys. Chem. Lett. 3, 2405–2408 (2012).
Shivanna, R. et al. Charge generation and transport in efficient organic bulk heterojunction solar cells with a perylene acceptor. Energy Environ. Sci. 7, 435–441 (2014).
Ye, L. et al. Enhanced efficiency in fullerene-free polymer solar cell by incorporating fine-designed donor and acceptor materials. ACS Appl. Mater. Interfaces 7, 9274–9280 (2015).
Liang, N. et al. Perylene diimide trimers based bulk heterojunction organic solar cells with efficiency over 7%. Adv. Energy Mater. 6, 1600060 (2016).
Jiang, W. et al. Bay-linked perylene bisimides as promising non-fullerene acceptors for organic solar cells. Chem. Commun. 50, 1024–1026 (2014).
Zang, Y. et al. Integrated molecular, interfacial, and device engineering towards high-performance non-fullerene based organic solar cells. Adv. Mater. 26, 5708–5714 (2014).
Ye, L. et al. Toward efficient non-fullerene polymer solar cells: selection of donor polymers. Org. Electron. 17, 295–303 (2015).
Wu, C. et al. Influence of molecular geometry of perylene diimide dimers and polymers on bulk heterojunction morphology toward high-performance nonfullerene polymer solar cells. Adv. Funct. Mater. 25, 5326–5332 (2015).
Sun, D. et al. Non-fullerene-acceptor-based bulk-heterojunction organic solar cells with efficiency over 7%. J. Am. Chem. Soc. 137, 11156–11162 (2015).
Meng, D. et al. High-performance solution-processed non-fullerene organic solar cells based on selenophene-containing perylene bisimide acceptor. J. Am. Chem. Soc. 138, 375–380 (2016).
Fan, Y. et al. Comparison of the optical and electrochemical properties of bi(perylene diimide)s linked through ortho and bay positions. ACS Omega 2, 377–385 (2017).
Yan, Q., Zhou, Y., Zheng, Y., Pei, J. & Zhao, D. Towards rational design of organic electron acceptors for photovoltaics: a study based on perylenediimide derivatives. Chem. Sci. 4, 4389–4394 (2013).
Zhang, X. et al. A potential perylene diimide dimer-based acceptor material for highly efficient solution-processed non-fullerene organic solar cells with 4.03% efficiency. Adv. Mater. 25, 5791–5797 (2013).
Lin, Y. et al. A star-shaped perylene diimide electron acceptor for high-performance organic solar cells. Adv. Mater. 26, 5137–5142 (2014).
Duan, Y. et al. Pronounced effects of a triazine core on photovoltaic performance-efficient organic solar cells enabled by a PDI trimer-based small molecular acceptor. Adv. Mater. 29, 1605115 (2017).
Lin, H. et al. Reduced intramolecular twisting improves the performance of 3D molecular acceptors in non-fullerene organic solar cells. Adv. Mater. 28, 8546–8551 (2016).
Wu, Q., Zhao, D., Schneider, A. M., Chen, W. & Yu, L. Covalently bound clusters of alpha-substituted PDI–rival electron acceptors to fullerene for organic solar cells. J. Am. Chem. Soc. 138, 7248–7251 (2016).
Chen, W. et al. A perylene diimide (PDI)-based small molecule with tetrahedral configuration as a non-fullerene acceptor for organic solar cells. J. Mater. Chem. C 3, 4698–4705 (2015).
Zhong, Y. et al. Efficient organic solar cells with helical perylene diimide electron acceptors. J. Am. Chem. Soc. 136, 15215–15221 (2014).
Zhong, Y. et al. Molecular helices as electron acceptors in high-performance bulk heterojunction solar cells. Nat. Commun. 6, 8242 (2015).
Zhong, H. et al. Rigidifying nonplanar perylene diimides by ring fusion toward geometry-tunable acceptors for high-performance fullerene-free solar cells. Adv. Mater. 28, 951–958 (2016).
Meng, D. et al. Three-bladed rylene propellers with three-dimensional network assembly for organic electronics. J. Am. Chem. Soc. 138, 10184–10190 (2016).
Facchetti, A. Polymer donor–polymer acceptor (all-polymer) solar cells. Mater. Today 16, 123–132 (2013).
Deshmukh, K. D. et al. Performance, morphology and photophysics of high open-circuit voltage, low band gap all-polymer solar cells. Energy Environ. Sci. 8, 332–342 (2015).
Lu, L. et al. Recent advances in bulk heterojunction polymer solar cells. Chem. Rev. 115, 12666–12731 (2015).
Moore, J. R. et al. Polymer blend solar cells based on a high-mobility naphthalenediimide-based polymer acceptor: device physics, photophysics and morphology. Adv. Energy Mater. 1, 230–240 (2011).
Guo, Y. et al. Improved performance of all-polymer solar cells enabled by naphthodiperylenetetraimide-based polymer acceptor. Adv. Mater. 29, 1700309 (2017).
Gao, L. et al. All-polymer solar cells based on absorption-complementary polymer donor and acceptor with high power conversion efficiency of 8.27%. Adv. Mater. 28, 1884–1890 (2016).
Fan, B. et al. Optimisation of processing solvent and molecular weight for the production of green-solvent-processed all-polymer solar cells with a power conversion efficiency over 9%. Energy Environ. Sci. 10, 1243–1251 (2017).
Zhan, X. et al. A high-mobility electron-transport polymer with broad absorption and its use in field-effect transistors and all-polymer solar cells. J. Am. Chem. Soc. 129, 7246–7247 (2007).
Tan, Z. et al. Efficient all-polymer solar cells based on blend of tris(thienylenevinylene)-substituted polythiophene and poly[perylene diimide-alt-bis(dithienothiophene)]. Appl. Phys. Lett. 93, 073309 (2008).
Zhan, X. et al. Copolymers of perylene diimide with dithienothiophene and dithienopyrrole as electron-transport materials for all-polymer solar cells and field-effect transistors. J. Mater. Chem. 19, 5794–5803 (2009).
Huang, J. et al. Photoinduced intramolecular electron transfer in conjugated perylene bisimide-dithienothiophene systems: a comparative study of a small molecule and a polymer. J. Phys. Chem. A 113, 5039–5046 (2009).
Cheng, P. et al. Binary additives synergistically boost the efficiency of all-polymer solar cells up to 3.45%. Energy Environ. Sci. 7, 1351–1356 (2014).
Cheng, P., Yan, C., Li, Y., Ma, W. & Zhan, X. Diluting concentrated solution: a general, simple and effective approach to enhance efficiency of polymer solar cells. Energy Environ. Sci. 8, 2357–2364 (2015).
Hou, J., Zhang, S., Chen, T. & Yang, Y. A new n-type low bandgap conjugated polymer P-co-CDT: synthesis and excellent reversible electrochemical and electrochromic properties. Chem. Commun. 6034–6036 (2008).
Zhou, E., Tajima, K., Yang, C. & Hashimoto, K. Band gap and molecular energy level control of perylene diimide-based donor–acceptor copolymers for all-polymer solar cells. J. Mater. Chem. 20, 2362–2368 (2010).
Zhou, E. et al. All-polymer solar cells from perylene diimide based copolymers: material design and phase separation control. Angew. Chem. Int. Ed. 50, 2799–2803 (2011).
Hu, X. et al. Synthesis and photovoltaic properties of n-type conjugated polymers alternating 2,7-carbazole and arylene diimides. Sol. Energy Mater. Sol. Cells 103, 157–163 (2012).
Zhou, Y. et al. New polymer acceptors for organic solar cells: the effect of regio-regularity and device configuration. J. Mater. Chem. A 1, 6609–6613 (2013).
Zhou, Y. et al. High performance all-polymer solar cell via polymer side-chain engineering. Adv. Mater. 26, 3767–3772 (2014).
Diao, Y. et al. Flow-enhanced solution printing of all-polymer solar cells. Nat. Commun. 6, 7955 (2015).
Guo, Y. et al. A vinylene-bridged perylenediimide-based polymeric acceptor enabling efficient all-polymer solar cells processed under ambient conditions. Adv. Mater. 28, 8483–8489 (2016).
Wurthner, F. et al. Preparation and characterization of regioisomerically pure 1,7-disubstituted perylene bisimide dyes. J. Org. Chem. 69, 7933–7939 (2004).
Yan, H. et al. A high-mobility electron-transporting polymer for printed transistors. Nature 457, 679–686 (2009).
Li, Z. et al. High performance all-polymer solar cells by synergistic effects of fine-tuned crystallinity and solvent annealing. J. Am. Chem. Soc. 138, 10935–10944 (2016).
Jung, J. et al. Fluoro-substituted n-type conjugated polymers for additive-free all-polymer bulk heterojunction solar cells with high power conversion efficiency of 6.71%. Adv. Mater. 27, 3310–3317 (2015).
Earmme, T., Hwang, Y., Murari, N. M., Subramaniyan, S. & Jenekhe, S. A. All-polymer solar cells with 3.3% efficiency based on naphthalene diimide-selenophene copolymer acceptor. J. Am. Chem. Soc. 135, 14960–14963 (2013).
Earmme, T., Hwang, Y., Subramaniyan, S. & Jenekhe, S. A. All-polymer bulk heterojuction solar cells with 4.8% efficiency achieved by solution processing from a co-solvent. Adv. Mater. 26, 6080–6085 (2014).
Hwang, Y., Courtright, B. A., Ferreira, A. S., Tolbert, S. H. & Jenekhe, S. A. 7.7% Efficient all-polymer solar cells. Adv. Mater. 27, 4578–4584 (2015).
Hwang, Y., Ren, G., Murari, N. M. & Jenekhe, S. A. n-Type naphthalene diimide–biselenophene copolymer for all-polymer bulk heterojunction solar cells. Macromolecules 45, 9056–9062 (2012).
Lee, C. et al. High-performance all-polymer solar cells via side-chain engineering of the polymer acceptor: the importance of the polymer packing structure and the nanoscale blend morphology. Adv. Mater. 27, 2466–2471 (2015).
Lee, W. et al. Side chain optimization of naphthalenediimide–bithiophene-based polymers to enhance the electron mobility and the performance in all-polymer solar cells. Adv. Funct. Mater. 26, 1543–1553 (2016).
Choi, J. et al. Importance of electron transport ability in naphthalene diimide-based polymer acceptors for high-performance, additive-free, all-polymer solar cells. Chem. Mater. 27, 5230–5237 (2015).
Hwang, Y., Earmme, T., Courtright, B. A., Eberle, F. N. & Jenekhe, S. A. n-Type semiconducting naphthalene diimide-perylene diimide copolymers: controlling crystallinity, blend morphology, and compatibility toward high-performance all-polymer solar cells. J. Am. Chem. Soc. 137, 4424–4434 (2015).
Wu, J., Cheng, S., Cheng, Y. & Hsu, C. Donor–acceptor conjugated polymers based on multifused ladder-type arenes for organic solar cells. Chem. Soc. Rev. 44, 1113–1154 (2015).
Han, G., Guo, Y., Song, X., Wang, Y. & Yi, Y. Terminal π–π stacking determines three-dimensional molecular packing and isotropic charge transport in an A–π–A electron acceptor for non-fullerene organic solar cells. J. Mater. Chem. C 5, 4852–4857 (2017).
Kim, Y., Song, C. E., Moon, S.-J. & Lim, E. Effect of dye end groups in non-fullerene fluorene- and carbazole-based small molecule acceptors on photovoltaic performance. RSC Adv. 5, 62739–62746 (2015).
Wang, K. et al. π-Bridge-independent 2-(benzo[c][1,2,5]thiadiazol-4-ylmethylene)malononitrile-substituted nonfullerene acceptors for efficient bulk heterojunction solar cells. Chem. Mater. 28, 2200–2208 (2016).
Zhang, G. et al. Efficient nonfullerene polymer solar cells enabled by a novel wide bandgap small molecular acceptor. Adv. Mater. 29, 1606054 (2017).
Stoltzfus, D. M., Clulow, A. J., Jin, H., Burn, P. L. & Gentle, I. R. Impact of dimerization on phase separation and crystallinity in bulk heterojunction films containing non-fullerene acceptors. Macromolecules 49, 4404–4415 (2016).
Lin, Y. et al. Structure evolution of oligomer fused-ring electron acceptors toward high efficiency of as-cast polymer solar cells. Adv. Energy Mater. 6, 1600854 (2016).
Kan, B. et al. Small-molecule acceptor based on the heptacyclic benzodi(cyclopentadithiophene) unit for highly efficient nonfullerene organic solar cells. J. Am. Chem. Soc. 139, 4929–4934 (2017).
Lin, Y. et al. An electron acceptor challenging fullerenes for efficient polymer solar cells. Adv. Mater. 27, 1170–1174 (2015).
Dai, S. et al. Fused nonacyclic electron acceptors for efficient polymer solar cells. J. Am. Chem. Soc. 139, 1336–1343 (2017).
Li, Y. et al. Non-fullerene acceptor with low energy loss and high external quantum efficiency: towards high performance polymer solar cells. J. Mater. Chem. A 4, 5890–5897 (2016).
Wang, W. et al. Fused hexacyclic nonfullerene acceptor with strong near-infrared absorption for semitransparent organic solar cells with 9.77% efficiency. Adv. Mater. 29, 1701308 (2017).
Zhao, W. et al. Fullerene-free polymer solar cells with over 11% efficiency and excellent thermal stability. Adv. Mater. 28, 4734–4739 (2016).
Bin, H. et al. 11.4% Efficiency non-fullerene polymer solar cells with trialkylsilyl substituted 2D-conjugated polymer as donor. Nat. Commun. 7, 13651 (2016).
Yang, Y. et al. Side-chain isomerization on an n-type organic semiconductor ITIC acceptor makes 11.77% high efficiency polymer solar cells. J. Am. Chem. Soc. 138, 15011–15018 (2016).
Zuo, L. et al. High-efficiency nonfullerene organic solar cells with a parallel tandem configuration. Adv. Mater. 29, 1702547 (2017).
Zhu, J. et al. Naphthodithiophene-based nonfullerene acceptor for high-performance organic photovoltaics: effect of extended conjugation. Adv. Mater. 30, 1704713 (2017).
Li, Y. et al. A fused-ring based electron acceptor for efficient non-fullerene polymer solar cells with small HOMO offset. Nano Energy 27, 430–438 (2016).
Kronenberg, N. M. et al. Bulk heterojunction organic solar cells based on merocyanine colorants. Chem. Commun. 6489–6491 (2008).
Wu, Y. et al. A planar electron acceptor for efficient polymer solar cells. Energy Environ. Sci. 8, 3215–3221 (2015).
Cheng, P. et al. Realizing small energy loss of 0.55 eV, high open-circuit voltage >1 V and high efficiency >10% in fullerene-free polymer solar cells via energy driver. Adv. Mater. 29, 1605216 (2017).
Xiao, B. et al. Achievement of high VOC of 1.02 V for P3HT-based organic solar cell using a benzotriazole-containing non-fullerene acceptor. Adv. Energy Mater. 7, 1602269 (2017).
Liu, F. et al. A thieno[3,4-b]thiophene-based non-fullerene electron acceptor for high-performance bulk-heterojunction organic solar cells. J. Am. Chem. Soc. 138, 15523–15526 (2016).
Jia, B. et al. Rhodanine flanked indacenodithiophene as non-fullerene acceptor for efficient polymer solar cells. Sci. China Chem. 60, 257–263 (2017).
Lin, Y. et al. A twisted dimeric perylene diimide electron acceptor for efficient organic solar cells. Adv. Energy Mater. 4, 1400420 (2014).
Li, S. et al. Energy-level modulation of small-molecule electron acceptors to achieve over 12% efficiency in polymer solar cells. Adv. Mater. 28, 9423–9429 (2016).
Yao, H. et al. Achieving highly efficient nonfullerene organic solar cells with improved intermolecular interaction and open-circuit voltage. Adv. Mater. 29, 1700254 (2017).
Xie, D. et al. A novel thiophene-fused ending group enabling an excellent small molecule acceptor for high-performance fullerene-free polymer solar cells with 11.8% efficiency. Sol. RRL 1, 1700044 (2017).
Feng, H. et al. An A-D-A type small-molecule electron acceptor with end-extended conjugation for high performance organic solar cells. Chem. Mater. 29, 7908–7917 (2017).
Lin, Y. et al. High-performance electron acceptor with thienyl side chains for organic photovoltaics. J. Am. Chem. Soc. 138, 4955–4961 (2016).
Zhao, F. et al. Single-junction binary-blend nonfullerene polymer solar cells with 12.1% efficiency. Adv. Mater. 29, 1700144 (2017).
Wang, J. et al. Enhancing performance of nonfullerene acceptors via side-chain conjugation strategy. Adv. Mater. 29, 1702125 (2017).
Lin, Y. et al. A facile planar fused-ring electron acceptor for as-cast polymer solar cells with 8.71% efficiency. J. Am. Chem. Soc. 138, 2973–2976 (2016).
Lin, Y. et al. Mapping polymer donors toward high-efficiency fullerene free organic solar cells. Adv. Mater. 29, 1604155 (2017).
Holliday, S. et al. High-efficiency and air-stable P3HT-based polymer solar cells with a new non-fullerene acceptor. Nat. Commun. 7, 11585 (2016).
Baran, D. et al. Reducing the efficiency–stability–cost gap of organic photovoltaics with highly efficient and stable small molecule acceptor ternary solar cells. Nat. Mater. 16, 363–369 (2017).
Lin, Y. et al. High-performance fullerene-free polymer solar cells with 6.31% efficiency. Energy Environ. Sci. 8, 610–616 (2015).
Bai, H. et al. An electron acceptor based on indacenodithiophene and 1,1-dicyanomethylene-3-indanone for fullerene-free organic solar cells. J. Mater. Chem. A 3, 1910–1914 (2015).
Yan, C. et al. Enhancing performance of non-fullerene organic solar cells via side chain engineering of fused-ring electron acceptors. Dyes Pigm. 139, 627–634 (2017).
Yao, H. et al. Design and synthesis of a low bandgap small molecule acceptor for efficient polymer solar cells. Adv. Mater. 28, 8283–8287 (2016).
Yao, H. et al. Design, synthesis, and photovoltaic characterization of a small molecular acceptor with an ultra-narrow band gap. Angew. Chem. Int. Ed. 56, 3045–3049 (2017).
Gao, L. et al. High-efficiency nonfullerene polymer solar cells with medium bandgap polymer donor and narrow bandgap organic semiconductor acceptor. Adv. Mater. 28, 8288–8295 (2016).
Bin, H. et al. Non-fullerene polymer solar cells based on alkylthio and fluorine substituted 2D-conjugated polymers reach 9.5% efficiency. J. Am. Chem. Soc. 138, 4657–4664 (2016).
Ye, L. et al. Manipulating aggregation and molecular orientation in all-polymer photovoltaic cells. Adv. Mater. 27, 6046–6054 (2015).
Yao, J. et al. Quantifying losses in open-circuit voltage in solution-processable solar cells. Phys. Rev. Appl. 4, 014020 (2015).
Scharber, M. C. et al. Design rules for donors in bulk-heterojunction solar cells — towards 10% energy-conversion efficiency. Adv. Mater. 18, 789–794 (2006).
Clarke, T. M. & Durrant, J. R. Charge photogeneration in organic solar cells. Chem. Rev. 110, 6736–6767 (2010).
Veldman, D., Meskers, S. C. J. & Janssen, R. A. J. The energy of charge-transfer states in electron donor–acceptor blends: insight into the energy losses in organic solar cells. Adv. Funct. Mater. 19, 1939–1948 (2009).
Li, W., Hendriks, K. H., Furlan, A., Wienk, M. M. & Janssen, R. A. High quantum efficiencies in polymer solar cells at energy losses below 0.6 eV. J. Am. Chem. Soc. 137, 2231–2234 (2015).
Kawashima, K., Tamai, Y., Ohkita, H., Osaka, I. & Takimiya, K. High-efficiency polymer solar cells with small photon energy loss. Nat. Commun. 6, 10085 (2015).
Ran, N. et al. Harvesting the full potential of photons with organic solar cells. Adv. Mater. 28, 1482–1488 (2016).
Wang, C. et al. Low band gap polymer solar cells with minimal voltage losses. Adv. Energy Mater. 6, 1600148 (2016).
Baran, D. et al. Reduced voltage losses yield 10% efficient fullerene free organic solar cells with >1 V open circuit voltages. Energy Environ. Sci. 9, 3783–3793 (2016).
Benduhn, J. et al. Intrinsic non-radiative voltage losses in fullerene-based organic solar cells. Nat. Energy 2, 17053 (2017).
Bartesaghi, D. et al. Competition between recombination and extraction of free charges determines the fill factor of organic solar cells. Nat. Commun. 6, 7083 (2015).
Collins, B. A. et al. Absolute measurement of domain composition and nanoscale size distribution explains performance in PTB7:PC71 BM solar cells. Adv. Energy Mater. 3, 65–74 (2013).
Liu, Y. et al. Aggregation and morphology control enables multiple cases of high-efficiency polymer solar cells. Nat. Commun. 5, 5293 (2014).
Mukherjee, S., Proctor, C. M., Bazan, G. C., Nguyen, T. Q. & Ade, H. Significance of average domain purity and mixed domains on the photovoltaic performance of high-efficiency solution-processed small-molecule BHJ solar cells. Adv. Energy Mater. 5, 1500877 (2015).
Jao, M., Liao, H. & Su, W. Achieving a high fill factor for organic solar cells. J. Mater. Chem. A 4, 5784–5801 (2016).
Xie, Z. & Wurthner, F. Hybrid photoconductive cathode interlayer materials composed of perylene bisimide photosensitizers and zinc oxide for high performance polymer solar cells. Adv. Energy Mater. 7, 1602573 (2017).
Cheng, P. & Zhan, X. Stability of organic solar cells: challenges and strategies. Chem. Soc. Rev. 45, 2544–2582 (2016).
Bai, H. et al. Nonfullerene acceptors based on extended fused rings flanked with benzothiadiazolylmethylenemalononitrile for polymer solar cells. J. Mater. Chem. A 3, 20758–20766 (2015).
Acknowledgements
X.Z. acknowledges support from the National Natural Science Foundation of China (Grant Nos 21734001 and 51761165023). S.B. and S.R.M. acknowledge support from the US Department of the Navy, Office of Naval Research (Grant No. N00014-14-1-0580 (CAOP MURI)). Z.W. acknowledges support from the National Natural Science Foundation of China (Grant No. 21734009). H.Y. acknowledges support from the National Basic Research Program of China (Grant Nos 2013CB834701 and 2014CB643501) and the Hong Kong Innovation and Technology Commission (Grant Nos ITC-CNERC14SC01 and ITS/083/15). A.K.-Y.J. acknowledges support from the US Office of Naval Research (Grant No. N00014-17-1-2201) and the Asian Office of Aerospace R&D (Grant No. FA2386-15-1-4106).
Author information
Authors and Affiliations
Contributions
C.Y., S.B., Z.W., H.Y. and X.Z. researched data for the article. All authors contributed to the writing and editing of the article before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Rights and permissions
About this article
Cite this article
Yan, C., Barlow, S., Wang, Z. et al. Non-fullerene acceptors for organic solar cells. Nat Rev Mater 3, 18003 (2018). https://doi.org/10.1038/natrevmats.2018.3
Published:
DOI: https://doi.org/10.1038/natrevmats.2018.3
This article is cited by
-
A highly crystalline face-on π-conjugated polymer based on alkoxythiophene-flanked benzobisthiazole for organic photovoltaics
Polymer Journal (2024)
-
Perovskite–organic tandem solar cells
Nature Reviews Materials (2024)
-
Assessing intra- and inter-molecular charge transfer excitations in non-fullerene acceptors using electroabsorption spectroscopy
Nature Communications (2024)
-
Accelerated discovery of molecular nanojunction photocatalysts for hydrogen evolution by using automated screening and flow synthesis
Nature Synthesis (2024)
-
Physical insights into non-fullerene organic photovoltaics
Nature Reviews Physics (2024)