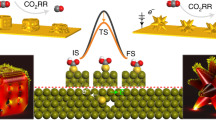
Abstract
Electrocatalysis is crucial for the development of clean and renewable energy technologies, which may reduce our reliance on fossil fuels. Multimetallic nanomaterials serve as state-of-the-art electrocatalysts as a consequence of their unique physico-chemical properties. One method of enhancing the electrocatalytic performance of multimetallic nanomaterials is to tune or control the surface strain of the nanomaterials, and tremendous progress has been made in this area in the past decade. In this Review, we summarize advances in the introduction, tuning and quantification of strain in multimetallic nanocrystals to achieve more efficient energy conversion by electrocatalysis. First, we introduce the concept of strain and its correlation with other key physico-chemical properties. Then, using the electrocatalytic reduction of oxygen as a model reaction, we discuss the underlying mechanisms behind the strain–adsorption–reactivity relationship based on combined classical theories and models. We describe how this knowledge can be harnessed to design multimetallic nanocrystals with optimized strain to increase the efficiency of oxygen reduction. In particular, we highlight the unexpectedly beneficial (and previously overlooked) role of tensile strain from multimetallic nanocrystals in improving electrocatalysis. We conclude by outlining the challenges and offering our perspectives on the research directions in this burgeoning field.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Gates, B. The energy research imperative. Science 334, 877 (2011).
Marban, G. & Valdes-Solis, T. Towards the hydrogen economy? Int. J. Hydrogen Energy 32, 1625–1637 (2007).
Yang, Z. et al. Electrochemical energy storage for green grid. Chem. Rev. 111, 3577–3613 (2011).
Bockris, J. O. M. The hydrogen economy: its history. Int. J. Hydrogen Energy 38, 2579–2588 (2013).
Lubitz, W. & Tumas, W. Hydrogen: an overview. Chem. Rev. 107, 3900–3903 (2007).
Chu, S. & Majumdar, A. Opportunities and challenges for a sustainable energy future. Nature 488, 294–303 (2012).
Groger, O., Gasteiger, H. A. & Suchsland, J.-P. Review—electromobility: batteries or fuel cells? J. Electrochem. Soc. 162, A2605–A2622 (2015).
Winter, M. & Brodd, R. J. What are batteries, fuel cells, and supercapacitors? Chem. Rev. 104, 4245–4270 (2004).
Lemmon, J. P. Energy: reimagine fuel cells. Nature 525, 447–449 (2015).
Debe, M. K. Electrocatalyst approaches and challenges for automotive fuel cells. Nature 486, 43–51 (2012).
Gasteiger, H. A. & Markovic, N. M. Just a dream or future reality? Science 324, 48–49 (2009).
Kongkanand, A. & Mathias, M. F. The priority and challenge of high-power performance of low-platinum proton-exchange membrane fuel cells. J. Phys. Chem. Lett. 7, 1127–1137 (2016).
Stephens, I. E., Rossmeisl, J. & Chorkendorff, I. Toward sustainable fuel cells. Science 354, 1378–1379 (2016).
Gasteiger, H. A., Kocha, S. S., Sompalli, B. & Wagner, F. T. Activity benchmarks and requirements for Pt, Pt-alloy, and non-Pt oxygen reduction catalysts for PEMFCs. Appl. Catal. B 56, 9–35 (2005).
Stephens, I. E. L., Bondarenko, A. S., Grønbjerg, U., Rossmeisl, J. & Chorkendorff, I. Understanding the electrocatalysis of oxygen reduction on platinum and its alloys. Energy Environ. Sci. 5, 6744–6762 (2012).
He, T., Kreidler, E., Xiong, L., Luo, J. & Zhong, C. J. Alloy electrocatalysts. J. Electrochem. Soc. 153, A1637–A1643 (2006).
Ferrando, R., Jellinek, J. & Johnston, R. L. Nanoalloys: from theory to applications of alloy clusters and nanoparticles. Chem. Rev. 108, 845–910 (2008).
Gilroy, K. D., Ruditskiy, A., Peng, H. C., Qin, D. & Xia, Y. Bimetallic nanocrystals: syntheses, properties, and applications. Chem. Rev. 116, 10414–11472 (2016).
Adzic, R. R. et al. Platinum monolayer fuel cell electrocatalysts. Top. Catal. 46, 249–262 (2007).
Adzic, R. R. Platinum monolayer electrocatalysts: tunable activity, stability, and self-healing properties. Electrocatalysis 3, 163–169 (2012).
Gan, L., Cui, C., Rudi, S. & Strasser, P. Core–shell and nanoporous particle architectures and their effect on the activity and stability of Pt ORR electrocatalysts. Top. Catal. 57, 236–244 (2013).
Yu, Z. et al. Comparison between dealloyed PtCo3 and PtCu3 cathode catalysts for proton exchange membrane fuel cells. J. Phys. Chem. C 116, 19877–19885 (2012).
Stamenkovic, V. R., Mun, B. S., Mayrhofer, K. J., Ross, P. N. & Markovic, N. M. Effect of surface composition on electronic structure, stability, and electrocatalytic properties of Pt-transition metal alloys: Pt-skin versus Pt-skeleton surfaces. J. Am. Chem. Soc. 128, 8813–8819 (2006).
Zhou, Z. Y., Tian, N., Li, J. T., Broadwell, I. & Sun, S. G. Nanomaterials of high surface energy with exceptional properties in catalysis and energy storage. Chem. Soc. Rev. 40, 4167–4185 (2011).
Wang, Y. J. et al. Carbon-supported Pt-based alloy electrocatalysts for the oxygen reduction reaction in polymer electrolyte membrane fuel cells: particle size, shape, and composition manipulation and their impact to activity. Chem. Rev. 115, 3433–3467 (2015).
Shao, M., Chang, Q., Dodelet, J. P. & Chenitz, R. Recent advances in electrocatalysts for oxygen reduction reaction. Chem. Rev. 116, 3594–3657 (2016).
Chen, Z., Waje, M., Li, W. & Yan, Y. Supportless Pt and PtPd nanotubes as electrocatalysts for oxygen-reduction reactions. Angew. Chem. Int. Ed. 119, 4138–4141 (2007).
Chen, C. et al. Highly crystalline multimetallic nanoframes with three-dimensional electrocatalytic surfaces. Science 343, 1339–1343 (2014).
Lim, B. et al. Pd-Pt bimetallic nanodendrites with high activity for oxygen reduction. Science 324, 1302–1305 (2009).
Zhang, L. et al. Platinum-based nanocages with subnanometer-thick walls and well-defined, controllable facets. Science 349, 412–416 (2015).
Guo, S. et al. FePt and CoPt nanowires as efficient catalysts for the oxygen reduction reaction. Angew. Chem. Int. Ed. 52, 3465–3468 (2013).
Strasser, P. Catalysts by platonic design. Science 349, 379–380 (2015).
Huang, X. Q. et al. High-performance transition metal-doped Pt3Ni octahedra for oxygen reduction reaction. Science 348, 1230–1234 (2015).
Stamenkovic, V. R. et al. Trends in electrocatalysis on extended and nanoscale Pt-bimetallic alloy surfaces. Nat. Mater. 6, 241–247 (2007).
Strasser, P. et al. Lattice-strain control of the activity in dealloyed core–shell fuel cell catalysts. Nat. Chem. 2, 454–460 (2010). This study rationalizes the strain effect on promoting the activities of ORRs on Pt. This is possible because of the isolation of the strain effect from the ensemble and ligand effects in the dealloyed catalysts.
Chen, M., Kumar, D., Yi, C. W. & Goodman, D. W. The promotional effect of gold in catalysis by palladium-gold. Science 310, 291–293 (2005).
Zhang, J., Vukmirovic, M. B., Xu, Y., Mavrikakis, M. & Adzic, R. R. Controlling the catalytic activity of platinum-monolayer electrocatalysts for oxygen reduction with different substrates. Angew. Chem. Int. Ed. 44, 2132–2135 (2005).
Mistry, H., Varela, A. S., Kühl, S., Strasser, P. & Cuenya, B. R. Nanostructured electrocatalysts with tunable activity and selectivity. Nat. Rev. Mater. 1, 16009 (2016).
Fiori, G. et al. Electronics based on two-dimensional materials. Nat. Nanotechnol. 9, 768–779 (2014).
Snedeker, J. G. et al. Strain-rate dependent material properties of the porcine and human kidney capsule. J. Biomech. 38, 1011–1021 (2005).
Wang, Y. et al. Super-elastic graphene ripples for flexible strain sensors. ACS Nano 5, 3645–3650 (2011).
Yang, S., Liu, F., Wu, C. & Yang, S. Tuning surface properties of low dimensional materials via strain engineering. Small 12, 4028–4047 (2016).
Parks, V. J. & Durelli, A. J. On the definitions of strain and their use in large-strain analysis. Exp. Mech. 7, 279–280 (1967).
Medasani, B. & Vasiliev, I. Computational study of the surface properties of aluminum nanoparticles. Surf. Sci. 603, 2042–2046 (2009).
Gilbert, B., Huang, F., Zhang, H., Waychunas, G. A. & Banfield, J. F. Nanoparticles: strained and stiff. Science 305, 651–654 (2004).
Wolfer, W. G. Elastic properties of surfaces on nanoparticles. Acta Mater. 59, 7736–7743 (2011).
Solliard, C. & Flueli, M. Surface stress and size effect on the lattice parameter in small particles of gold and platinum. Surf. Sci. 156, 487–494 (1985).
Potapenko, D. V., Li, Z., Kysar, J. W. & Osgood, R. M. Nanoscale strain engineering on the surface of a bulk TiO2 crystal. Nano Lett. 14, 6185–6189 (2014).
Gsell, M., Jakob, P. & Menzel, D. Effect of substrate strain on adsorption. Science 280, 717–720 (1998). Direct evidence for the effect of strain on adsorption properties.
Kato, H., Tottori, Y. & Sasaki, K. Four-point bending test of determining stress-strain curves asymmetric between tension and compression. Exp. Mech. 54, 489–492 (2013).
Castellanos-Gomez, A. et al. Local strain engineering in atomically thin MoS2 . Nano Lett. 13, 5361–5366 (2013).
Du, M., Cui, L., Cao, Y. & Bard, A. J. Mechanoelectrochemical catalysis of the effect of elastic strain on a platinum nanofilm for the ORR exerted by a shape memory alloy substrate. J. Am. Chem. Soc. 137, 7397–7403 (2015).
Wang, H. et al. Direct and continuous strain control of catalysts with tunable battery electrode materials. Science 354, 1031–1036 (2016).
Ohzuku, T. & Ueda, A. Solid-state redox reactions of LiCoO2 (R3_m) for 4 volt secondary lithium cells. J. Electrochem. Soc. 141, 2972–2977 (1994).
Shao-Horn, Y., Croguennec, L., Delmas, C., Nelson, E. C. & O’Keefe, M. A. Atomic resolution of lithium ions in LiCoO2 . Nat. Mater. 2, 464–467 (2003).
Strasser, P. & Kühl, S. Dealloyed Pt-based core–shell oxygen reduction electrocatalysts. Nano Energy 29, 166–177 (2016).
Yang, H. Platinum-based electrocatalysts with core–shell nanostructures. Angew. Chem. Int. Ed. 50, 2674–2676 (2011).
Guo, S., Zhang, S. & Sun, S. Tuning nanoparticle catalysis for the oxygen reduction reaction. Angew. Chem. Int. Ed. 52, 8526–8544 (2013).
Sneed, B. T., Young, A. P. & Tsung, C. K. Building up strain in colloidal metal nanoparticle catalysts. Nanoscale 7, 12248–12265 (2015).
Korte, C., Peters, A., Janek, J., Hesse, D. & Zakharov, N. Ionic conductivity and activation energy for oxygen ion transport in superlattices—the semicoherent multilayer system YSZ (ZrO2 + 9.5 mol% Y2O3)/Y2O3 . Phys. Chem. Chem. Phys. 10, 4623–4635 (2008).
Zeng, J. et al. Controlling the nucleation and growth of silver on palladium nanocubes by manipulating the reaction kinetics. Angew. Chem. Int. Ed. 51, 2354–2358 (2012).
Lim, B. et al. Facile synthesis of bimetallic nanoplates consisting of Pd cores and Pt shells through seeded epitaxial growth. Nano Lett. 8, 2535–2540 (2008).
Jiang, M. et al. Epitaxial overgrowth of platinum on palladium nanocrystals. Nanoscale 2, 2406–2411 (2010).
Wang, D., Zhao, P. & Li, Y. General preparation for Pt-based alloy nanoporous nanoparticles as potential nanocatalysts. Sci. Rep. 1, 37–42 (2011).
Srivastava, R., Mani, P., Hahn, N. & Strasser, P. Efficient oxygen reduction fuel cell electrocatalysis on voltammetrically dealloyed Pt–Cu–Co nanoparticles. Angew. Chem. Int. Ed. 46, 8988–8991 (2007).
Hasché, F., Oezaslan, M. & Strasser, P. Activity, stability, and degradation mechanisms of dealloyed PtCu3 and PtCo3 nanoparticle fuel cell catalysts. ChemCatChem 3, 1805–1813 (2011).
Zhang, J. et al. Mixed-metal Pt monolayer electrocatalysts for enhanced oxygen reduction kinetics. J. Am. Chem. Soc. 127, 12480–12481 (2005).
Zhou, W. P. et al. Improving electrocatalysts for O2 reduction by fine-tuning the Pt-support interaction: Pt monolayer on the surfaces of a Pd3Fe(111) single-crystal alloy. J. Am. Chem. Soc. 131, 12755–12762 (2009).
Zhang, J. et al. Platinum monolayer electrocatalysts for O2 reduction: Pt monolayer on Pd(111) and on carbon-supported Pd nanoparticles. J. Phys. Chem. B 108, 10955–10964 (2004).
Stamenkovic, V. R. et al. Improved oxygen reduction activity on Pt3Ni(111) via increased surface site availability. Science 315, 493–497 (2007).
Stamenkovic, V., Schmidt, T. J., Ross, P. N. & Markovic, N. M. Surface composition effects in electrocatalysis: kinetics of oxygen reduction on well-defined Pt3Ni and Pt3Co alloy surfaces. J. Phys. Chem. B 106, 11970–11979 (2002).
Leonardi, A., Leoni, M., Siboni, S. & Scardi, P. Common volume functions and diffraction line profiles of polyhedral domains. J. Appl. Crystallogr. 45, 1162–1172 (2012).
Huang, W. J. et al. Coordination-dependent surface atomic contraction in nanocrystals revealed by coherent diffraction. Nat. Mater. 7, 308–313 (2008). This study shows the dependence of surface strain on the coordination numbers and exposed facets of the nanoparticulate system.
Wu, J. et al. Surface lattice-engineered bimetallic nanoparticles and their catalytic properties. Chem. Soc. Rev. 41, 8066–8074 (2012).
Miao, J., Ohsuna, T., Terasaki, O., Hodgson, K. O. & O’Keefe, M. A. Atomic resolution three-dimensional electron diffraction microscopy. Phys. Rev. Lett. 89, 155502 (2002).
Kim, S. et al. 3D strain measurement in electronic devices using through-focal annular dark-field imaging. Ultramicroscopy 146, 1–5 (2014).
Miao, J., Ercius, P. & Billinge, S. J. Atomic electron tomography: 3D structures without crystals. Science 353, 1380–1388 (2016).
Xu, R. et al. Three-dimensional coordinates of individual atoms in materials revealed by electron tomography. Nat. Mater. 14, 1099–1103 (2015).
Goris, B. et al. Atomic-scale determination of surface facets in gold nanorods. Nat. Mater. 11, 930–935 (2012).
Kibler, L. A., El-Aziz, A. M., Hoyer, R. & Kolb, D. M. Tuning reaction rates by lateral strain in a palladium monolayer. Angew. Chem. Int. Ed. 44, 2080–2084 (2005).
Koh, S. & Strasser, P. Electrocatalysis on bimetallic surfaces: modifying catalytic reactivity for oxygen reduction by voltammetric surface dealloying. J. Am. Chem. Soc. 129, 12624–12625 (2007).
Muralidharan, N., Carter, R., Oakes, L., Cohn, A. P. & Pint, C. L. Strain engineering to modify the electrochemistry of energy storage electrodes. Sci. Rep. 6, 27542–27551 (2016).
Markovic, N. M. Electrocatalysis: interfacing electrochemistry. Nat. Mater. 12, 101–102 (2013).
Stamenkovic, V. R., Strmcnik, D., Lopes, P. P. & Markovic, N. M. Energy and fuels from electrochemical interfaces. Nat. Mater. 16, 57–69 (2016).
Trasatti, S. Work function, electronegativity, and electrochemical behaviour of metals: III. Electrolytic hydrogen evolution in acid solutions. J. Electroanal. Chem. 39, 163–184 (1972).
Medford, A. J. et al. From the Sabatier principle to a predictive theory of transition-metal heterogeneous catalysis. J. Catal. 328, 36–42 (2015).
Stamenkovic, V. et al. Changing the activity of electrocatalysts for oxygen reduction by tuning the surface electronic structure. Angew. Chem. Int. Ed. 45, 2897–2901 (2006).
Sheng, W., Myint, M., Chen, J. G. & Yan, Y. Correlating the hydrogen evolution reaction activity in alkaline electrolytes with the hydrogen binding energy on monometallic surfaces. Energy Environ. Sci. 6, 1509–1512 (2013).
Jalan, V. & Taylor, E. J. Importance of interatomic spacing in catalytic reduction of oxygen in phosphoric acid. J. Electrochem. Soc. 130, 2299–2302 (1983). This study shows the correlation between the activities of the ORR with interatomic distances in Pt-based alloys.
Mavrikakis, M., Hammer, B. & Nørskov, J. K. Effect of strain on the reactivity of metal surfaces. Phys. Rev. Lett. 81, 2819–2822 (1998). Using the d-band model, this work correlates surface strain with the adsorption properties and hence the reactivity of late transition metals.
Hammer, B. & Nørskov, J. K. Electronic factors determining the reactivity of metal surfaces. Surf. Sci. 343, 211–220 (1995).
Schnur, S. & Groß, A. Strain and coordination effects in the adsorption properties of early transition metals: a density-functional theory study. Phys. Rev. B 81, 033402 (2010). Using the d-band model, this work correlates surface strain with the adsorption properties of ETMs.
Jia, Q. et al. Activity descriptor identification for oxygen reduction on platinum-based bimetallic nanoparticles: in situ observation of the linear composition–strain–activity relationship. ACS Nano 9, 387–400 (2015).
Moseley, P. & Curtin, W. A. Computational design of strain in core–shell nanoparticles for optimizing catalytic activity. Nano Lett. 15, 4089–4095 (2015).
Temmel, S. E., Fabbri, E., Pergolesi, D., Lippert, T. & Schmidt, T. J. Investigating the role of strain toward the oxygen reduction activity on model thin film Pt catalysts. ACS Catal. 6, 7566–7576 (2016).
Li, M. et al. Ultrafine jagged platinum nanowires enable ultrahigh mass activity for the oxygen reduction reaction. Science 354, 1414–1419 (2016).
Fortunelli, A. et al. The atomistic origin of the extraordinary oxygen reduction activity of Pt3Ni7 fuel cell catalysts. Chem. Sci. 6, 3915–3925 (2015).
Debe, M. K. et al. Extraordinary oxygen reduction activity of Pt3Ni7 . J. Electrochem. Soc. 158, B910–B918 (2011).
Yang, J., Chen, X., Yang, X. & Ying, J. Y. Stabilization and compressive strain effect of AuCu core on Pt shell for oxygen reduction reaction. Energy Environ. Sci. 5, 8976–8981 (2012).
Erlebacher, J., Aziz, M. J., Karma, A., Dimitrov, N. & Sieradzki, K. Evolution of nanoporosity in dealloying. Nature 410, 450–453 (2001).
Gan, L., Heggen, M., Rudi, S. & Strasser, P. Core–shell compositional fine structures of dealloyed PtxNi1 − x nanoparticles and their impact on oxygen reduction catalysis. Nano Lett. 12, 5423–5430 (2012).
Rudi, S., Tuaev, X. & Strasser, P. Electrocatalytic oxygen reduction on dealloyed Pt1− xNix alloy nanoparticle electrocatalysts. Electrocatalysis 3, 265–273 (2012).
Neyerlin, K. C., Srivastava, R., Yu, C. & Strasser, P. Electrochemical activity and stability of dealloyed Pt–Cu and Pt–Cu–Co electrocatalysts for the oxygen reduction reaction (ORR). J. Power Sources 186, 261–267 (2009).
Luo, M., Wei, L., Wang, F., Han, K. & Zhu, H. Gram-level synthesis of core–shell structured catalysts for the oxygen reduction reaction in proton exchange membrane fuel cells. J. Power Sources 270, 34–41 (2014).
Gan, L., Yu, R., Luo, J., Cheng, Z. & Zhu, J. Lattice strain distributions in individual dealloyed Pt–Fe catalyst nanoparticles. J. Phys. Chem. Lett. 3, 934–938 (2012). This study was the first to map strain distribution on a single dealloyed nanoparticle.
Han, B. et al. Record activity and stability of dealloyed bimetallic catalysts for proton exchange membrane fuel cells. Energy Environ. Sci. 8, 258–266 (2015). This work reports a strain-engineered catalyst that achieved the world-record activity in practical fuel cell operation.
Gan, L., Heggen, M., O’Malley, R., Theobald, B. & Strasser, P. Understanding and controlling nanoporosity formation for improving the stability of bimetallic fuel cell catalysts. Nano Lett. 13, 1131–1138 (2013).
Wang, C. et al. Design and synthesis of bimetallic electrocatalyst with multilayered Pt-skin surfaces. J. Am. Chem. Soc. 133, 14396–14403 (2011).
Wang, D. et al. Structurally ordered intermetallic platinum–cobalt core–shell nanoparticles with enhanced activity and stability as oxygen reduction electrocatalysts. Nat. Mater. 12, 81–87 (2013).
Zhang, S. et al. Tuning nanoparticle structure and surface strain for catalysis optimization. J. Am. Chem. Soc. 136, 7734–7739 (2014).
Greeley, J. et al. Alloys of platinum and early transition metals as oxygen reduction electrocatalysts. Nat. Chem. 1, 552–556 (2009).
Escudero-Escribano, M. et al. Pt5Gd as a highly active and stable catalyst for oxygen electroreduction. J. Am. Chem. Soc. 134, 16476–16479 (2012).
Malacrida, P., Escudero-Escribano, M., Verdaguer-Casadevall, A., Stephens, I. E. L. & Chorkendorff, I. Enhanced activity and stability of Pt–La and Pt–Ce alloys for oxygen electroreduction: the elucidation of the active surface phase. J. Mater. Chem. A 2, 4234–4243 (2014).
Johansson, T. P. et al. Pt skin versus Pt skeleton structures of Pt3Sc as electrocatalysts for oxygen reduction. Top. Catal. 57, 245–254 (2013).
Velázquez-Palenzuela, A. et al. The enhanced activity of mass-selected PtxGd nanoparticles for oxygen electroreduction. J. Catal. 328, 297–307 (2015).
Hernandez-Fernandez, P. et al. Mass-selected nanoparticles of PtxY as model catalysts for oxygen electroreduction. Nat. Chem. 6, 732–738 (2014).
Johansson, T. P. et al. Towards the elucidation of the high oxygen electroreduction activity of PtxY: surface science and electrochemical studies of Y/Pt(111). Phys. Chem. Chem. Phys. 16, 13718–13725 (2014).
Escudero-Escribano, M. et al. Tuning the activity of Pt alloy electrocatalysts by means of the lanthanide contraction. Science 352, 73–76 (2016).
Bian, T. et al. Epitaxial growth of twinned Au–Pt core–shell star-shaped decahedra as highly durable electrocatalysts. Nano Lett. 15, 7808–7815 (2015).
Wu, J. et al. Icosahedral platinum alloy nanocrystals with enhanced electrocatalytic activities. J. Am. Chem. Soc. 134, 11880–11883 (2012).
Zhang, Z. et al. One-pot synthesis of highly anisotropic five-fold-twinned PtCu nanoframes used as a bifunctional electrocatalyst for oxygen reduction and methanol oxidation. Adv. Mater. 28, 8712–8717 (2016).
Wang, X. et al. Pt-based icosahedral nanocages: using a combination of {111} facets, twin defects, and ultrathin walls to greatly enhance their activity toward oxygen reduction. Nano Lett. 16, 1467–1471 (2016).
Sun, X., Jiang, K., Zhang, N., Guo, S. & Huang, X. Crystalline control of {111} bounded Pt3Cu nanocrystals: multiply-twinned Pt3Cu icosahedra with enhanced electrocatalytic properties. ACS Nano 9, 7634–7640 (2015).
Ghosh, T., Vukmirovic, M. B., DiSalvo, F. J. & Adzic, R. R. Intermetallics as novel supports for Pt monolayer O2 reduction electrocatalysts: potential for significantly improving properties. J. Am. Chem. Soc. 132, 906–907 (2010).
Liu, F., Wu, C., Yang, G. & Yang, S. CO oxidation over strained Pt(100) surface: a DFT study. J. Phys. Chem. C 119, 15500–15505 (2015).
Francis, M. F. & Curtin, W. A. Mechanical work makes important contributions to surface chemistry at steps. Nat. Commun. 6, 6261–6268 (2015).
Bu, L. et al. Biaxially strained PtPb/Pt core/shell nanoplate boosts oxygen reduction catalysis. Science 354, 1410–1414 (2016). This study shows that the large tensile strain of the Pt(110) facet promotes oxygen reduction catalysis.
Shao, M. Palladium-based electrocatalysts for hydrogen oxidation and oxygen reduction reactions. J. Power Sources 196, 2433–2444 (2011).
Shao, M., Liu, P., Zhang, J. & Adzic, R. Origin of enhanced activity in palladium alloy electrocatalysts for oxygen reduction reaction. J. Phys. Chem. B. 111, 6772–6775 (2007).
Guo, S. et al. Nanocatalyst superior to Pt for oxygen reduction reactions: the case of core/shell Ag(Au)/CuPd nanoparticles. J. Am. Chem. Soc. 136, 15026–15033 (2014).
Jiang, K. et al. Ordered PdCu-based nanoparticles as bifunctional oxygen-reduction and ethanol-oxidation electrocatalysts. Angew. Chem. Int. Ed. 55, 9030–9035 (2016).
Meku, E. et al. Electrocatalytic activity and stability of ordered intermetallic palladium-iron nanoparticles toward oxygen reduction reaction. J. Electrochem. Soc. 163, F132–F138 (2016).
Jiang, G. et al. Core/shell face-centered tetragonal FePd/Pd nanoparticles as an efficient non-Pt catalyst for the oxygen reduction reaction. ACS Nano 9, 11014–11022 (2015).
Chen, Z. et al. Multiscale computational design of core/shell nanoparticles for oxygen reduction reaction. J. Phys. Chem. C 121, 1964–1973 (2017).
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (NSFC) (Grant No. 51671003), the China Postdoctoral Science Foundation (Grant No. 2017M610022), the National Basic Research Program of China (Grant No. 2016YFB0100201), the Open Project Foundation of the State Key Laboratory of Chemical Resource Engineering, and start-up support from Peking University and the Young Thousand Talents Program.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Rights and permissions
About this article
Cite this article
Luo, M., Guo, S. Strain-controlled electrocatalysis on multimetallic nanomaterials. Nat Rev Mater 2, 17059 (2017). https://doi.org/10.1038/natrevmats.2017.59
Published:
DOI: https://doi.org/10.1038/natrevmats.2017.59
This article is cited by
-
Electrocatalytic hydrogenation of acetonitrile to ethylamine in acid
Nature Communications (2024)
-
A Prospective on Energy and Environment Applications of High Entropy Alloys
Transactions of the Indian National Academy of Engineering (2024)
-
Strain engineering in electrocatalysis: Strategies, characterization, and insights
Nano Research (2024)
-
Ultra-Efficient and Cost-Effective Platinum Nanomembrane Electrocatalyst for Sustainable Hydrogen Production
Nano-Micro Letters (2024)
-
Deformable Catalytic Material Derived from Mechanical Flexibility for Hydrogen Evolution Reaction
Nano-Micro Letters (2024)