Abstract
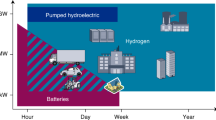
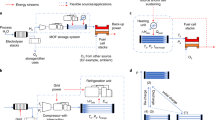
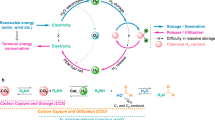
Hydrogen has the potential to be a major energy vector in a renewable and sustainable future energy mix. The efficient production, storage and delivery of hydrogen are key technical issues that require improvement before its potential can be realized. In this Review, we focus on recent advances in materials development for on-board hydrogen storage. We highlight the strategic design and optimization of hydrides of light-weight elements (for example, boron, nitrogen and carbon) and physisorbents (for example, metal–organic and covalent organic frameworks). Furthermore, hydrogen carriers (for example, NH3, CH3OH–H2O and cycloalkanes) for large-scale distribution and for on-site hydrogen generation are discussed with an emphasis on dehydrogenation catalysts.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Schlapbach, L. & Züttel, A. Hydrogen-storage materials for mobile applications. Nature 414, 353–358 (2001).
Orimo, S.-i., Nakamori, Y., Eliseo, J. R., Züttel, A. & Jensen, C. M. Complex hydrides for hydrogen storage. Chem. Rev. 107, 4111–4132 (2007).
Eberle, U., Felderhoff, M. & Schuth, F. Chemical and physical solutions for hydrogen storage. Angew. Chem. Int. Ed. 48, 6608–6630 (2009).
Yang, J., Sudik, A., Wolverton, C. & Siegel, D. J. High capacity hydrogen storage materials: attributes for automotive applications and techniques for materials discovery. Chem. Soc. Rev. 39, 656–675 (2010).
Klerke, A., Christensen, C. H., Norskov, J. K. & Vegge, T. Ammonia for hydrogen storage: challenges and opportunities. J. Mater. Chem. 18, 2304–2310 (2008).
Crabtree, R. H. Hydrogen storage in liquid organic heterocycles. Energy Environ. Sci. 1, 134–138 (2008).
Palo, D. R., Dagle, R. A. & Holladay, J. D. Methanol steam reforming for hydrogen production. Chem. Rev. 107, 3992–4021 (2007).
Zhu, Q.-L. & Xu, Q. Liquid organic and inorganic chemical hydrides for high-capacity hydrogen storage. Energy Environ. Sci. 8, 478–512 (2015).
Pasini, J. M. et al. Metal hydride material requirements for automotive hydrogen storage systems. Int. J. Hydrogen Energy 38, 9755–9765 (2013).
Züttel, A. et al. LiBH4 a new hydrogen storage material. J. Power Sources 118, 1–7 (2003). LiBH4, now one of the most intensively studied hydrides, was investigated for the first time in this paper as a H2 storage material.
Chen, P., Xiong, Z., Luo, J., Lin, J. & Tan, K. L. Interaction of hydrogen with metal nitrides and imides. Nature 420, 302–304 (2002). This is the pioneering work on amide hydrides for H2 storage, which further initiated research into reactive composite systems for H2 storage.
Gutowska, A. et al. Nanoscaffold mediates hydrogen release and the reactivity of ammonia borane. Angew. Chem. Int. Ed. 44, 3578–3582 (2005). This work demonstrated that confining ammonia borane in a nanoporous SBA-15 scaffold led to significantly improved dehydrogenation properties, and thus, stimulated research into nanoconfinement.
Biniwale, R. B., Rayalu, S., Devotta, S. & Ichikawa, M. Chemical hydrides: a solution to high capacity hydrogen storage and supply. Int. J. Hydrogen Energy 33, 360–365 (2008).
Rosi, N. L. et al. Hydrogen storage in microporous metal–organic frameworks. Science 300, 1127–1129 (2003). This is the pioneering work in which MOFs were first used as an efficient physisorbent for storing H2.
Bogdanović, B. & Schwickardi, M. Ti-doped alkali metal aluminium hydrides as potential novel reversible hydrogen storage materials. J. Alloys Compd. 253–254, 1–9 (1997).
Pez, G. P., Scott, A. R., Cooper, A. C. & Cheng, H. Hydrogen storage by reversible hydrogenation of pi-conjugated substrates. US patent 7101530 (2006). This patent demonstrated for the first time that heterocyclic compounds possess better thermodynamic properties than their hydrocarbon counterparts and thus show promise for on-board applications.
Grochala, W. & Edwards, P. P. Thermal decomposition of the non-interstitial hydrides for the storage and production of hydrogen. Chem. Rev. 104, 1283–1316 (2004).
Chlopek, K., Frommen, C., Léon, A., Zabara, O. & Fichtner, M. Synthesis and properties of magnesium tetrahydroborate, Mg(BH4)2 . J. Mater. Chem. 17, 3496–3503 (2007).
Xiong, Z. et al. High-capacity hydrogen storage in lithium and sodium amidoboranes. Nat. Mater. 7, 138–141 (2008).
Diyabalanage, H. V. K. et al. Calcium amidotrihydroborate: a hydrogen storage material. Angew. Chem. Int. Ed. 46, 8995–8997 (2007).
Wu, H. et al. Metal hydrazinoborane LiN2H3BH3 and LiN2H3BH3•2N2H4BH3: crystal structures and high-extent dehydrogenation. Energy Environ. Sci. 5, 7531–7535 (2012).
Chen, J. et al. Lithiated primary amine — a new material for hydrogen storage. Chem. Eur. J. 20, 6632–6635 (2014).
Nickels, E. A. et al. Tuning the decomposition temperature in complex hydrides: synthesis of a mixed alkali metal borohydride. Angew. Chem. Int. Ed. 47, 2817–2819 (2008).
Hagemann, H. et al. LiSc(BH4)4: a novel salt of Li+ and discrete Sc(BH4)4− complex anions. J. Phys. Chem. A 112, 7551–7555 (2008).
Wu, H. et al. Sodium magnesium amidoborane: the first mixed-metal amidoborane. Chem. Commun. 47, 4102–4104 (2011).
Orimo, S.-I. et al. Experimental studies on intermediate compound of LiBH4 . Appl. Phys. Lett. 89, 021920 (2006).
Li, H. W. et al. Effects of ball milling and additives on dehydriding behaviors of well-crystallized Mg(BH4)2 . Scr. Mater. 57, 679–682 (2007).
Soloveichik, G. L. et al. Magnesium borohydride as a hydrogen storage material: properties and dehydrogenation pathway of unsolvated Mg(BH4)2 . Int. J. Hydrogen Energy 34, 916–928 (2009).
Ravnsbaek, D. et al. A series of mixed-metal borohydrides. Angew. Chem. Int. Ed. 48, 6659–6663 (2009).
Kim, D. Y., Singh, N. J., Lee, H. M. & Kim, K. S. Hydrogen-release mechanisms in lithium amidoboranes. Chem. Euro. J. 15, 5598–5604 (2009).
Akbarzadeh, A. R., Ozolins, V. & Wolverton, C. First-principles determination of multicomponent hydride phase diagrams: application to the Li-Mg-N-H system. Adv. Mater. 19, 3233–3239 (2007).
Alapati, S. V., Johnson, J. K. & Sholl, D. S. Identification of destabilized metal hydrides for hydrogen storage using first principles calculations. J. Phys. Chem. B 110, 8769–8776 (2006).
Vajo, J. J., Skeith, S. L. & Mertens, F. Reversible storage of hydrogen in destabilized LiBH4 . J. Phys. Chem. B 109, 3719–3722 (2005). This work demonstrated that the compositing of 2LiBH4 and MgH2 resulted in significantly improved thermodynamics for H2 storage.
Xiong, Z., Wu, G., Hu, J. & Chen, P. Ternary imides for hydrogen storage. Adv. Mater. 16, 1522–1525 (2004).
Luo, W. F. (LiNH2–MgH2): a viable hydrogen storage system. J. Alloys Compd. 381, 284–287 (2004).
Leng, H. Y. et al. New metal-N-H system composed of Mg(NH2)2 and LiH for hydrogen storage. J. Phys. Chem. B 108, 8763–8765 (2004).
Bosenberg, U. et al. Hydrogen sorption properties of MgH2–LiBH4 composites. Acta Mater. 55, 3951–3958 (2007).
Soloveichik, G. et al. Ammine magnesium borohydride complex as a new material for hydrogen storage: structure and properties of mg(BH4)2·2NH3 . Inorg. Chem. 47, 4290–4298 (2008).
He, T. et al. Borohydride hydrazinates: high hydrogen content materials for hydrogen storage. Energy Environ. Sci. 5, 5686–5689 (2012).
Chua, Y. S. et al. Synthesis, structure and dehydrogenation of magnesium amidoborane monoammoniate. Chem. Commun. 46, 5752–5754 (2010).
Guo, Y., Yu, X., Sun, W., Sun, D. & Yang, W. The hydrogen-enriched Al–B–N system as an advanced solid hydrogen-storage candidate. Angew. Chem. Int. Ed. 50, 1087–1091 (2011).
Luo, W., Zakharov, L. N. & Liu, S.-Y. 1,2-BN cyclohexane: synthesis, structure, dynamics, and reactivity. J. Am. Chem. Soc. 133, 13006–13009 (2011).
Wu, H., Zhou, W. & Yildirim, T. Alkali and alkaline-earth metal amidoboranes: structure, crystal chemistry, and hydrogen storage properties. J. Am. Chem. Soc. 130, 14834–14839 (2008).
Diyabalanage, H. V. K. et al. Potassium(I) amidotrihydroborate: structure and hydrogen release. J. Am. Chem. Soc. 132, 11836–11837 (2010).
Chua, Y. S. et al. Alkali metal hydride modification on hydrazine borane for improved dehydrogenation. J. Phys. Chem. C 118, 11244–11251 (2014).
Nakamori, Y. et al. Correlation between thermodynamical stabilities of metal borohydrides and cation electronegativites: first-principles calculations and experiments. Phys. Rev. B 74, 045126 (2006).
Graetz, J. New approaches to hydrogen storage. Chem. Soc. Rev. 38, 73–82 (2009).
Schouwink, P. et al. Structure and properties of complex hydride perovskite materials. Nat. Commun. 5, 5706 (2014).
Cerny, R. et al. AZn2(BH4)5 (A = Li, Na) and NaZn(BH4)3: structural studies. J. Phys. Chem. C 114, 19127–19133 (2010).
Filinchuk, Y. et al. Porous and dense magnesium borohydride frameworks: synthesis, stability, and reversible absorption of guest species. Angew. Chem. Int. Ed. 50, 11162–11166 (2011).
Rude, L. H. et al. Synthesis and structural investigation of Zr(BH4)4 . J. Phys. Chem. C 116, 20239–20245 (2012).
Kang, X., Luo, J., Zhang, Q. & Wang, P. Combined formation and decomposition of dual-metal amidoborane NaMg(NH2BH3)3 for high-performance hydrogen storage. Dalton Trans. 40, 3799–3801 (2011).
Fijalkowski, K. J. et al. Na[Li(NH2BH3)2] — the first mixed-cation amidoborane with unusual crystal structure. Dalton Trans. 40, 4407–4413 (2011).
Udovic, T. J. et al. Exceptional superionic conductivity in disordered sodium decahydro-closo-decaborate. Adv. Mater. 26, 7622–7626 (2014).
Unemoto, A., Matsuo, M. & Orimo, S.-i. Complex hydrides for electrochemical energy storage. Adv. Funct. Mater. 24, 2267–2279 (2014).
Matsuo, M. & Orimo, S.-i. Lithium fast-ionic conduction in complex hydrides: review and prospects. Adv. Energy Mater. 1, 161–172 (2011).
David, W. I. F. et al. A mechanism for non-stoichiometry in the lithium amide/lithium imide hydrogen storage reaction. J. Am. Chem. Soc. 129, 1594–1601 (2007).
Rijssenbeek, J. et al. Crystal structure determination and reaction pathway of amide–hydride mixtures. J. Alloys Compd. 454, 233–244 (2008).
Wu, H. Structure of ternary imide Li2Ca(NH)2 and hydrogen storage mechanisms in amide–hydride system. J. Am. Chem. Soc. 130, 6515–6522 (2008).
Verdal, N., Udovic, T. J., Rush, J. J., Wu, H. & Skripov, A. V. Evolution of the reorientational motions of the tetrahydroborate anions in hexagonal LiBH4–Lil solid solution by high-Q quasielastic neutron scattering. J. Phys. Chem. C 117, 12010–12018 (2013).
Rude, L. H. et al. Bromide substitution in lithium borohydride, LiBH4–LiBr. Int. J. Hydrogen Energy 36, 15664–15672 (2011).
Wu, H. et al. A new family of metal borohydride ammonia borane complexes: synthesis, structures, and hydrogen storage properties. J. Mater. Chem. 20, 6550–6556 (2010).
Luo, J., Wu, H., Zhou, W., Kang, X. & Wang, P. Li2(NH2BH3)(BH4)/LiNH2BH3: the first metal amidoborane borohydride complex with inseparable amidoborane precursor for hydrogen storage. Int. J. Hydrogen Energy 38, 197–204 (2013).
Gu, Q. et al. Structure and decomposition of zinc borohydride ammonia adduct: towards a pure hydrogen release. Energy Environ. Sci. 5, 7590–7600 (2012).
Kang, X., Wu, H., Luo, J., Zhou, W. & Wang, P. A simple and efficient approach to synthesize amidoborane ammoniates: case study for Mg(NH2BH3)2(NH3)3 with unusual coordination structure. J. Mater. Chem. 22, 13174–13179 (2012).
He, T. et al. Nanosized Co- and Ni-catalyzed ammonia borane for hydrogen storage. Chem. Mater. 21, 2315–2318 (2009).
Yan, J.-M., Zhang, X.-B., Han, S., Shioyama, H. & Xu, Q. Iron-nanoparticle-catalyzed hydrolytic dehydrogenation of ammonia borane for chemical hydrogen storage. Angew. Chem. Int. Ed. 120, 2319–2321 (2008).
Pinkerton, F. E., Meyer, M. S., Meisner, G. P. & Balogh, M. P. Improved hydrogen release from LiB0.33N0.67H2.67 with metal additives: Ni, Fe, and Zn. J. Alloys Compd. 433, 282–291 (2007).
Singh, S. K. & Xu, Q. Complete conversion of hydrous hydrazine to hydrogen at room temperature for chemical hydrogen storage. J. Am. Chem. Soc. 131, 18032–18033 (2009).
Denney, M. C., Pons, V., Hebden, T. J., Heinekey, D. M. & Goldberg, K. I. Efficient catalysis of ammonia borane dehydrogenation. J. Am. Chem. Soc. 128, 12048–12049 (2006).
Wang, Z., Tonks, I., Belli, J. & Jensen, C. M. Dehydrogenation of N-ethyl perhydrocarbazole catalyzed by PCP pincer iridium complexes: evaluation of a homogenous hydrogen storage system. J. Organomet. Chem. 694, 2854–2857 (2009).
Au, M. & Jurgensen, A. Modified lithium borohydrides for reversible hydrogen storage. J. Phys. Chem. B 110, 7062–7067 (2006).
Callini, E., Borgschulte, A., Hugelshofer, C. L., Ramirez-Cuesta, A. J. & Zuettel, A. The role of Ti in alanates and borohydrides: catalysis and metathesis. J. Phys. Chem. C 118, 77–84 (2014).
Bosenberg, U. et al. Role of additives in LiBH4–MgH2 reactive hydride composites for sorption kinetics. Acta Mater. 58, 3381–3389 (2010).
Wang, J. et al. Potassium-modified Mg(NH2)2/2LiH system for hydrogen storage. Angew. Chem. Int. Ed. 48, 5828–5832 (2009).
Li, C., Liu, Y., Gu, Y., Gao, M. & Pan, H. Improved hydrogen-storage thermodynamics and kinetics for an RbF-doped Mg(NH2)2–2LiH system. Chem. Asian J. 8, 2136–2143 (2013).
Sakintuna, B., Lamari-Darkrim, F. & Hirscher, M. Metal hydride materials for solid hydrogen storage: a review. Int. J. Hydrogen Energy 32, 1121–1140 (2007).
Liu, Y. et al. Size-dependent kinetic enhancement in hydrogen absorption and desorption of the Li-Mg-N-H system. J. Am. Chem. Soc. 131, 1862–1870 (2009).
de Jongh, P. E., Allendorf, M., Vajo, J. J. & Zlotea, C. Nanoconfined light metal hydrides for reversible hydrogen storage. MRS Bull. 38, 488–494 (2013).
Nielsen, T. K., Besenbacher, F. & Jensen, T. R. Nanoconfined hydrides for energy storage. Nanoscale 3, 2086–2098 (2011).
Gross, A. F., Vajo, J. J., Van Atta, S. L. & Olson, G. L. Enhanced hydrogen storage kinetics of LiBH4 in nanoporous carbon scaffolds. J. Phys. Chem. C 112, 5651–5657 (2008).
Feaver, A. et al. Coherent carbon cryogel–ammonia borane nanocomposites for H2 storage. J. Phys. Chem. B 111, 7469–7472 (2007).
Li, Z., Zhu, G., Lu, G., Qiu, S. & Yao, X. Ammonia borane confined by a metal–organic framework for chemical hydrogen storage: enhancing kinetics and eliminating ammonia. J. Am. Chem. Soc. 132, 1490–1491 (2010).
Zhao, J. et al. A soft hydrogen storage material: poly(methyl acrylate)-confined ammonia borane with controllable dehydrogenation. Adv. Mater. 22, 394–397 (2010).
Fang, Z. Z. et al. Kinetic- and thermodynamic-based improvements of lithium borohydride incorporated into activated carbon. Acta Mater. 56, 6257–6263 (2008).
Nielsen, T. K. et al. A reversible nanoconfined chemical reaction. ACS Nano 4, 3903–3908 (2010).
Demir-Cakan, R., Tang, W. S., Darwiche, A & Janot, R. Modification of the hydrogen storage properties of Li3N by confinement into mesoporous carbons. Energy Environ. Sci. 4, 3625–3631 (2011).
Ngene, P. et al. The role of Ni in increasing the reversibility of the hydrogen release from nanoconfined LiBH4 . Faraday Discuss. 151, 47–58 (2011).
Zhou, H.-C., Long, J. R. & Yaghi, O. M. Introduction to metal–organic frameworks. Chem. Rev. 112, 673–674 (2012).
Kitagawa, S. & Uemura, K. Dynamic porous properties of coordination polymers inspired by hydrogen bonds. Chem. Soc. Rev. 34, 109–119 (2005).
Farha, O. K. et al. Metal–organic framework materials with ultrahigh surface areas: is the sky the limit? J. Am. Chem. Soc. 134, 15016–15021 (2012).
Xu, Y., Jin, S., Xu, H., Nagai, A. & Jiang, D. Conjugated microporous polymers: design, synthesis and application. Chem. Soc. Rev. 42, 8012–8031 (2013).
Ding, S.-Y. & Wang, W. Covalent organic frameworks (COFs): from design to applications. Chem. Soc. Rev. 42, 548–568 (2013).
Ströbel, R., Garche, J., Moseley, P. T., Jörissen, L. & Wolf, G. Hydrogen storage by carbon materials. J. Power Sources 159, 781–801 (2006).
Bae, Y.-S. & Snurr, R. Q. Optimal isosteric heat of adsorption for hydrogen storage and delivery using metal–organic frameworks. Micropor. Mesopor. Mater. 132, 300–303 (2010).
Murray, L. J., Dinca, M. & Long, J. R. Hydrogen storage in metal–organic frameworks. Chem. Soc. Rev. 38, 1294–1314 (2009).
Ferey, G. et al. Hydrogen adsorption in the nanoporous metal-benzenedicarboxylate M(OH)(O2C–C6H4–CO2) (M = Al3+, Cr3+), MIL-53. Chem. Commun. 2003, 2976–2977 (2003).
Wang, Z., Tanabe, K. K. & Cohen, S. M. Tuning hydrogen sorption properties of metal–organic frameworks by postsynthetic covalent modification. Chem. Euro. J. 16, 212–217 (2010).
Rowsell, J. L. C., Millward, A. R., Park, K. S. & Yaghi, O. M. Hydrogen sorption in functionalized metal–organic frameworks. J. Am. Chem. Soc. 126, 5666–5667 (2004).
Park, K. S. et al. Exceptional chemical and thermal stability of zeolitic imidazolate frameworks. Proc. Natl Acad. Sci. USA 103, 10186–10191 (2006).
Wong-Foy, A. G., Matzger, A. J. & Yaghi, O. M. Exceptional H2 saturation uptake in microporous metal–organic frameworks. J. Am. Chem. Soc. 128, 3494–3495 (2006).
Lin, X. et al. High H2 adsorption by coordination-framework materials. Angew. Chem. Int. Ed. 45, 7358–7364 (2006).
Yan, Y., Yang, S., Blake, A. J. & Schröder, M. Studies on metal–organic frameworks of Cu(II) with isophthalate linkers for hydrogen storage. Acc. Chem. Res. 47, 296–307 (2014).
Sumida, K., Hill, M. R., Horike, S., Dailly, A. & Long, J. R. Synthesis and hydrogen storage properties of Be12(OH)12(1,3,5-benzenetribenzoate)4 . J. Am. Chem. Soc. 131, 15120–15121 (2009).
He, Y. & Chen, B. in Encyclopedia of Inorganic and Bioinorganic Chemistry (John Wiley & Sons, 2011).
Kapelewski, M. T. et al. M2(m-dobdc) (M = Mg, Mn, Fe, Co, Ni) metal–organic frameworks exhibiting increased charge density and enhanced H2 binding at the open metal sites. J. Am. Chem. Soc. 136, 12119–12129 (2014). The contribution of unsaturated metal sites for increased H2 binding enthalpies and close packing of H2 molecules within the framework are summarized in this article, along with the first-principles electronic structure calculations elucidating how the subtle structural and electronic differences give rise to increased H2 binding enthalpies.
Xiao, B. et al. High-capacity hydrogen and nitric oxide adsorption and storage in a metal–organic framework. J. Am. Chem. Soc. 129, 1203–1209 (2007).
Rousseau, R. et al. Defining active catalyst structure and reaction pathways from ab initio molecular dynamics and operando XAFS: dehydrogenation of dimethylaminoborane by rhodium clusters. J. Am. Chem. Soc. 131, 10516–10524 (2009).
Lee, Y.-G., Moon, H. R., Cheon, Y. E. & Suh, M. P. A comparison of the H2 sorption capacities of isostructural metal–organic frameworks with and without accessible metal sites: [{Zn2(abtc)(dmf)2}3] and [{Cu2(abtc)(dmf)2}3] versus [{Cu2(abtc)}3]. Angew. Chem. Int. Ed. 47, 7741–7745 (2008).
Dincaˇ, M. et al. Hydrogen storage in a microporous metal–organic framework with exposed Mn2+ coordination sites. J. Am. Chem. Soc. 128, 16876–16883 (2006).
Mueller, U. et al. Metal–organic frameworks-prospective industrial applications. J. Mater. Chem. 16, 626–636 (2006).
Belof, J. L., Stern, A. C., Eddaoudi, M. & Space, B. On the mechanism of hydrogen storage in a metal–organic framework material. J. Am. Chem. Soc. 129, 15202–15210 (2007).
Ryan, P., Broadbelt, L. J. & Snurr, R. Q. Is catenation beneficial for hydrogen storage in metal–organic frameworks? Chem. Commun. 2008, 4132–4134 (2008).
Ma, S. et al. Framework-catenation isomerism in metal–organic frameworks and its impact on hydrogen uptake. J. Am. Chem. Soc. 129, 1858–1859 (2007).
Pachfule, P., Chen, Y., Jiang, J. & Banerjee, R. Experimental and computational approach of understanding the gas adsorption in amino functionalized interpenetrated metal organic frameworks (MOFs). J. Mater. Chem. 21, 17737–17745 (2011).
Liu, X. et al. A twofold interpenetrating porous metal–organic framework with high hydrothermal stability: structure and gas sorption behavior. Inorg. Chem. 48, 11507–11509 (2009).
Kim, H. et al. Synthesis of phase-pure interpenetrated MOF-5 and its gas sorption properties. Inorg. Chem. 50, 3691–3696 (2011).
Yang, S. et al. Enhancement of H2 adsorption in coordination framework materials by use of ligand curvature. Chem. Euro. J. 15, 4829–4835 (2009).
Hulvey, Z., Falcao, E. H. L., Eckert, J. & Cheetham, A. K. Enhanced H2 adsorption enthalpy in the low-surface area, partially fluorinated coordination polymer Zn5(triazole)6(tetrafluoroterephthalate)2(H2O)2·4H2O. J. Mater. Chem. 19, 4307–4309 (2009).
Yang, C., Wang, X. & Omary, M. A. Fluorous metal–organic frameworks for high-density gas adsorption. J. Am. Chem. Soc. 129, 15454–15455 (2007).
Pan, L. et al. Microporous metal organic materials: promising candidates as sorbents for hydrogen storage. J. Am. Chem. Soc. 126, 1308–1309 (2004).
Klontzas, E., Mavrandonakis, A., Tylianakis, E. & Froudakis, G. E. Improving hydrogen storage capacity of MOF by functionalization of the organic linker with lithium atoms. Nano Lett. 8, 1572–1576 (2008).
Himsl, D., Wallacher, D. & Hartmann, M. Improving the hydrogen-adsorption properties of a hydroxy-modified MIL-53(Al) structural analogue by lithium doping. Angew. Chem. Int. Ed. 48, 4639–4642 (2009).
Lim, D.-W., Chyun, S. A. & Suh, M. P. Hydrogen storage in a potassium-ion-bound metal–organic framework incorporating crown ether struts as specific cation binding sites. Angew. Chem. Int. Ed. 126, 7953–7956 (2014).
Mulfort, K. L., Farha, O. K., Stern, C. L., Sarjeant, A. A. & Hupp, J. T. Post-synthesis alkoxide formation within metal–organic framework materials: a strategy for incorporating highly coordinatively unsaturated metal ions. J. Am. Chem. Soc. 131, 3866–3868 (2009).
Yang, S. et al. Enhancement of H2 adsorption in Li+-exchanged co-ordination framework materials. Chem. Commun. 2008, 6108–6110 (2008).
Han, S. S. & Goddard, W. A. Lithium-doped metal–organic frameworks for reversible H2 storage at ambient temperature. J. Am. Chem. Soc. 129, 8422–8423 (2007).
Miller, M. A., Wang, C.-Y. & Merrill, G. N. Experimental and theoretical investigation into hydrogen storage via spillover in IRMOF-8. J. Phys. Chem. C 113, 3222–3231 (2009).
Cheon, Y. E. & Suh, M. P. Enhanced hydrogen storage by palladium nanoparticles fabricated in a redox-active metal–organic framework. Angew. Chem. Int. Ed. 48, 2899–2903 (2009).
Contescu, C. I., Brown, C. M., Liu, Y., Bhat, V. V. & Gallego, N. C. Detection of hydrogen spillover in palladium-modified activated carbon fibers during hydrogen adsorption. J. Phys. Chem. C 113, 5886–5890 (2009).
Li, Y. & Yang, R. T. Hydrogen storage in metal–organic frameworks by bridged hydrogen spillover. J. Am. Chem. Soc. 128, 8136–8137 (2006).
Zlotea, C. et al. Pd nanoparticles embedded into a metal–organic framework: synthesis, structural characteristics, and hydrogen sorption properties. J. Am. Chem. Soc. 132, 2991–2997 (2010).
Liu, S. S. et al. Gold supported on titania for specific monohydrogenation of dinitroaromatics in the liquid phase. Green Chem. 16, 4162–4169 (2014).
Campesi, R., Cuevas, F., Latroche, M. & Hirscher, M. Hydrogen spillover measurements of unbridged and bridged metal–organic frameworks-revisited. Phys. Chem. Chem. Phys. 12, 10457–10459 (2010).
Szilagyi, P. A. et al. Probing hydrogen spillover in Pd@MIL-101(Cr) with a focus on hydrogen chemisorption. Phys. Chem. Chem. Phys. 16, 5803–5809 (2014).
Yuan, D., Lu, W., Zhao, D. & Zhou, H.-C. Highly stable porous polymer networks with exceptionally high gas-uptake capacities. Adv. Mater. 23, 3723–3725 (2011).
Ben, T. et al. Targeted synthesis of a porous aromatic framework with high stability and exceptionally high surface area. Angew. Chem. Int. Ed. 121, 9621–9624 (2009).
Jiang, J.-X. et al. Conjugated microporous poly(phenylene butadiynylene)s. Chem. Commun. 2008, 486–488 (2008).
Yuan, S. et al. Microporous polyphenylenes with tunable pore size for hydrogen storage. Chem. Commun. 46, 4547–4549 (2010).
Colson, J. W. & Dichtel, W. R. Rationally synthesized two-dimensional polymers. Nat. Chem. 5, 453–465 (2013).
Furukawa, H. & Yaghi, O. M. Storage of hydrogen, methane, and carbon dioxide in highly porous covalent organic frameworks for clean energy applications. J. Am. Chem. Soc. 131, 8875–8883 (2009).
Han, S. S., Furukawa, H., Yaghi, O. M. & Goddard, W. A. Covalent organic frameworks as exceptional hydrogen storage materials. J. Am. Chem. Soc. 130, 11580–11581 (2008).
Kuhn, P., Forget, A., Su, D., Thomas, A. & Antonietti, M. From microporous regular frameworks to mesoporous materials with ultrahigh surface area: dynamic reorganization of porous polymer networks. J. Am. Chem. Soc. 130, 13333–13337 (2008).
Kuhn, P., Antonietti, M. & Thomas, A. Porous, covalent triazine-based frameworks prepared by ionothermal synthesis. Angew. Chem. Int. Ed. 47, 3450–3453 (2008). This article details the synthesis and applications of nitrogen-rich covalent triazine framework for H2 absorption.
Wan, S. et al. Covalent organic frameworks with high charge carrier mobility. Chem. Mater. 23, 4094–4097 (2011).
Kandambeth, S. et al. Construction of crystalline 2D covalent organic frameworks with remarkable chemical (acid/base) stability via a combined reversible and irreversible route. J. Am. Chem. Soc. 134, 19524–19527 (2012).
Cao, D., Lan, J., Wang, W. & Smit, B. Lithium-doped 3D covalent organic frameworks: high-capacity hydrogen storage materials. Angew. Chem. Int. Ed. 48, 4730–4733 (2009).
Kalidindi, S. B. et al. Metal@COFs: covalent organic frameworks as templates for Pd nanoparticles and hydrogen storage properties of Pd@COF-102 hybrid material. Chem. Eur. J. 18, 10848–10856 (2012).
Züttel, A. Materials for hydrogen storage. Mater. Today 6, 24–33 (2003).
Titirici, M.-M. et al. Sustainable carbon materials. Chem. Soc. Rev. 44, 250–290 (2015).
Sevilla, M. & Mokaya, R. Energy storage applications of activated carbons: supercapacitors and hydrogen storage. Energy Environ. Sci. 7, 1250–1280 (2014).
Ryoo, R., Joo, S. H., Kruk, M. & Jaroniec, M. Ordered mesoporous carbons. Adv. Mater. 13, 677–681 (2001).
Nishihara, H. & Kyotani, T. Templated nanocarbons for energy storage. Adv. Mater. 24, 4473–4498 (2012).
Liu, B., Shioyama, H., Akita, T. & Xu, Q. Metal–organic framework as a template for porous carbon synthesis. J. Am. Chem. Soc. 130, 5390–5391 (2008).
Aijaz, A., Akita, T., Yang, H. & Xu, Q. From ionic-liquid@metal–organic framework composites to heteroatom-decorated large-surface area carbons: superior CO2 and H2 uptake. Chem. Commun. 50, 6498–6501 (2014).
Zhu, Z. H., Lu, G. Q. & Hatori, H. New insights into the interaction of hydrogen atoms with boron-substituted carbon. J. Phys. Chem. B 110, 1249–1255 (2006).
Wang, L. & Yang, R. T. New sorbents for hydrogen storage by hydrogen spillover — a review. Energy Environ. Sci. 1, 268–279 (2008).
Gomez, D. A. & Sastre, G. From microscopic insights of H2 adsorption to uptake estimations in MOFs. Phys. Chem. Chem. Phys. 13, 16558–16568 (2011).
Boddien, A. et al. Efficient dehydrogenation of formic acid using an iron catalyst. Science 333, 1733–1736 (2011).
Xu, J. G. & Froment, G. F. Methane steam reforming, methanation and water-gas shift: I. Intrinsic kinetics. Aiche J. 35, 88–96 (1989).
Schueth, F., Palkovits, R., Schloegl, R. & Su, D. S. Ammonia as a possible element in an energy infrastructure: catalysts for ammonia decomposition. Energy Environ. Sci. 5, 6278–6289 (2012).
Norskov, J. K., Bligaard, T., Rossmeisl, J. & Christensen, C. H. Towards the computational design of solid catalysts. Nat. Chem. 1, 37–46 (2009).
Nørskov, J. K. & Bligaard, T. The catalyst genome. Angew. Chem. Int. Ed. 52, 776–777 (2013).
Guo, J. et al. Lithium imide synergy with 3d transition-metal nitrides leading to unprecedented catalytic activities for ammonia decomposition. Angew. Chem. Int. Ed. 54, 2950–2954 (2015).
Guo, X. G. et al. Direct, nonoxidative conversion of methane to ethylene, aromatics, and hydrogen. Science 344, 616–619 (2014).
Ingleson, M. F. et al. Magnesium borohydride confined in a metal–organic framework: a preorganized system for facile arene hydroboration. Angew. Chem. Int. Ed. 48, 2012–2016 (2009).
Hartman, M. R., Rush, J. J., Udovic, T. J., Bowman, R. C. Jr & Hwang, S.-J. Structure and vibrational dynamics of isotopically labeled lithium borohydride using neutron diffraction and spectroscopy. J. Solid State Chem. 180, 1298–1305 (2007).
Černý, R., Filinchuk, Y., Hagemann, H. & Yvon, K. Magnesium borohydride: synthesis and crystal Structure. Angew. Chem. Int. Ed. 46, 5765–5767 (2007).
Wu, H., Zhou, W., Udovic, T. J., Rush, J. J. & Yildirim, T. Structures and crystal chemistry of Li2BNH6 and Li4BN3H10 . Chem. Mater. 20, 1245–1247 (2008).
He, T. et al. Lithium amidoborane hydrazinates: synthesis, structure and hydrogen storage properties. J. Mater. Chem. A 3, 10100–10106 (2015).
Stowe, A. C., Shaw, W. J., Linehan, J. C., Schmid, B. & Autrey, T. In situ solid state 11B MAS-NMR studies of the thermal decomposition of ammonia borane: mechanistic studies of the hydrogen release pathways from a solid state hydrogen storage material. Phys. Chem. Chem. Phys. 9, 1831–1836 (2007).
Bluhm, M. E., Bradley, M. G., Butterick, R., Kusari, U. & Sneddon, L. G. Amineborane-based chemical hydrogen storage: enhanced ammonia borane dehydrogenation in ionic liquids. J. Am. Chem. Soc. 128, 7748–7749 (2006).
Keaton, R. J., Blacquiere, J. M. & Baker, R. T. Base metal catalyzed dehydrogenation of ammonia borane for chemical hydrogen storage. J. Am. Chem. Soc. 129, 1844–1845 (2007).
Jaska, C. A., Temple, K., Lough, A. J. & Manners, I. Rhodium-catalyzed formation of boron-nitrogen bonds: a mild route to cyclic aminoboranes and borazines. Chem. Commun. 2001, 962–963 (2001).
Stephens, F. H., Baker, R. T., Matus, M. H., Grant, D. J. & Dixon, D. A. Acid initiation of ammonia-borane dehydrogenation for hydrogen Storage. Angew. Chem. Int. Ed. 46, 746–749 (2007).
Himmelberger, D. W., Yoon, C. W., Bluhm, M. E., Carroll, P. J. & Sneddon, L. G. Base-promoted ammonia borane hydrogen-release. J. Am. Chem. Soc. 131, 14101–14110 (2009).
Acknowledgements
This work is financially supported by the Project of National Science Funds for Distinguished Young Scholars (51225206), the Collaborative Innovation Center of Chemistry for Energy Materials and the Youth Innovation Promotion Association (CAS) of China and Ministry of Economy, Trade and Industry (METI) of Japan.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Rights and permissions
About this article
Cite this article
He, T., Pachfule, P., Wu, H. et al. Hydrogen carriers. Nat Rev Mater 1, 16059 (2016). https://doi.org/10.1038/natrevmats.2016.59
Published:
DOI: https://doi.org/10.1038/natrevmats.2016.59
This article is cited by
-
Highly loaded bimetallic iron-cobalt catalysts for hydrogen release from ammonia
Nature Communications (2024)
-
Cellulose fibers/polydopamine/zinc sulfide composite paper: predoping in situ fabrication for biphase interface photocatalytic hydrogen production from water
Cellulose (2024)
-
Quantitative Risk Assessment of a Liquid Organic Hydrogen Carriers-Based Hydrogen Refueling Station
Korean Journal of Chemical Engineering (2024)
-
Layered niobium carbide enabling excellent kinetics and cycling stability of Li-Mg-B-H hydrogen storage material
Rare Metals (2024)
-
Single Ti atoms coupled with Ti–O clusters enable low temperature hydrogen cycling by sodium alanate
Rare Metals (2024)