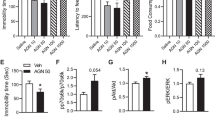
Abstract
Conventional antidepressant medications, which act on monoaminergic systems, display significant limitations, including a time lag of weeks to months and low rates of therapeutic efficacy. GLYX-13 is a novel glutamatergic compound that acts as an N-methyl-d-aspartate (NMDA) modulator with glycine-like partial agonist properties; like the NMDA receptor antagonist ketamine GLYX-13 produces rapid antidepressant actions in depressed patients and in preclinical rodent models. However, the mechanisms underlying the antidepressant actions of GLYX-13 have not been characterized. Here we use a combination of neutralizing antibody (nAb), mutant mouse and pharmacological approaches to test the role of brain-derived neurotrophic factor-tropomyosin-related kinase B (BDNF-TrkB) signaling in the actions of GLYX-13. The results demonstrate that the antidepressant effects of GLYX-13 are blocked by intra-medial prefrontal cortex (intra-mPFC) infusion of an anti-BDNF nAb or in mice with a knock-in of the BDNF Val66Met allele, which blocks the processing and activity-dependent release of BDNF. We also demonstrate that pharmacological inhibitors of BDNF-TrkB signaling or of l-type voltage-dependent Ca2+ channels (VDCCs) block the antidepressant behavioral actions of GLYX-13. Finally, we examined the role of the Rho GTPase proteins by injecting a selective inhibitor into the mPFC and found that activation of Rac1 but not RhoA is involved in the antidepressant effects of GLYX-13. Together, these findings indicate that enhanced release of BDNF through exocytosis caused by activation of VDCCs and subsequent TrkB-Rac1 signaling is required for the rapid and sustained antidepressant effects of GLYX-13.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Kessler RC, Chiu WC, Demler O, Walters F. Prevalence, severity and co-morbidity of 12 month DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry 2005; 62: 617–627.
Fava M. Diagnosis and definition of treatment-resistant depression. Biol Psychiatry 2003; 15: 649–659.
Rush AJ, Fava M, Wisniewski SR, Lavori PW, Trivedi MH, Sackeim HA et al. Sequenced treatment alternatives to relieve depression (STAR*D): rationale and design. Control Clin Trials 2004; 25: 119–142.
Trivedi MH, Rush AJ, Wisniewski SR, Nierenberg AA, Warden D, Ritz L et al. Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: implications for clinical practice. Am J Psychiatry 2006; 163: 28–40.
Altamura CA, Mauri MC, Ferrara A, Moro AR, D'Andrea G, Zamberlan F. Plasma and platelet excitatory amino acids in psychiatric disorders. Am J Psychiatry 1993; 150: 1731–1733.
Mauri MC, Ferrara A, Boscati L, Bravin S, Zamberlan F, Alecci M et al. Plasma and platelet amino acid concentrations in patients affected by major depression and under fluvoxamine treatment. Neuropsychobiology 1998; 37: 124–129.
Mitani H, Shirayama Y, Yamada T, Maeda K, Ashby CR Jr, Kawahara R. Correlation between plasma levels of glutamate, alanine and serine with severity of depression. Prog Neuropsychopharmacol Biol Psychiatry 2006; 30: 1155–1158.
Abdallah CG, Sanacora G, Duman RS, Krystal JH. Ketamine and rapid-acting antidepressants: a window into a new neurobiology for mood disorder therapeutics. Annu Rev Med 2015; 66: 509–523.
Berman RM, Cappiello A, Anand A, Oren DA, Heninger GR, Charney DS et al. Antidepressant effects of ketamine in depressed patients. Biol Psychiatry 2000; 47: 351–354.
Krystal JH, Karper LP, Seibyl JP, Freeman GK, Delaney R, Bremner D et al. Subanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humans: psychotomimetic, perceptual, cognitive, and neuroendocrine responses. Arch Gen Psychiatry 1994; 51: 199–214.
Lahti AC, Koffel B, LaPorte D, Tamminga CA. Subanesthetic doses of ketamine stimulate psychosis in schizophrenia. Neuropsychopharmacology 1995; 13: 9–19.
Malhotra AK, Pinals DA, Adler CM, Elman I, Clifton A, Pickar D et al. Ketamine-induced exacerbation of psychotic symptoms and cognitive impairment in neuroleptic-free schizophrenics. Neuropsychopharmacology 1997; 17: 141–150.
Moskal JR, Kuo AG, Weiss C, Wood PL, O'Connor Hanson A, Kelso S et al. GLYX-13: a monoclonal antibody-derived peptide that acts as an N-methyl-D-aspartate receptor modulator. Neuropharmacology 2005; 49: 1077–1087.
Zhang XL, Sullivan JA, Moskal JR, Stanton PK. A NMDA receptor glycine site partial agonist, GLYX-13, simultaneously enhances LTP and reduces LTD at Schaffer collateral-CA1 synapses in hippocampus. Neuropharmacology 2008; 55: 1238–1250.
Preskorn S, Macaluso M, Mehra DO, Zammit G, Moskal JR, Burch RM et al. Randomized proof of concept trial of GLYX-13, an N-methyl-D-aspartate receptor glycine site partial agonist, in major depressive disorder nonresponsive to a previous antidepressant agent. J Psychiatr Pract 2015; 21: 140–149.
Burgdorf J, Zhang XL, Weiss C, Gross A, Boikess SR, Kroes RA et al. The long-lasting antidepressant effects of rapastinel (GLYX-13) are associated with a metaplasticity process in the medial prefrontal cortex and hippocampus. Neuroscience 2015; 12: 202–211.
Liu RJ, Duman C, Kato T, Hare B, Lopresto D, Bang E et al. GLYX-13 produces rapid antidepressant responses with key synaptic and behavioral effects distinct from ketamine. Neuropsychopharmacology 2016; 42: 1231–1242.
Liu RJ, Lee FS, Li XY, Bambico F, Duman RS, Aghajanian GK. Brain-derived neurotrophic factor Val66Met allele impairs basal and ketamine-stimulated synaptogenesis in prefrontal cortex. Biol Psychiatry 2012; 71: 996–1005.
Lepack AE, Fuchikami M, Dwyer JM, Banasr M, Duman RS. BDNF release is required for the behavioral actions of ketamine. Int J Neuropsychopharmacol 2014; 18: 1–6.
Lepack AE, Bang E, Lee B, Dwyer JM, Duman RS. Fast-acting antidepressants rapidly stimulate ERK signaling and BDNF release in primary neuronal cultures. Neuropharmacology 2016; 111: 242–252.
Autry AE, Adachi M, Nosyreva E, Na ES, Los MF, Cheng PF et al. NMDA receptor blockade at rest triggers rapid behavioural antidepressant responses. Nature 2011; 15: 91–95.
Hedrick NG, Harward SC, Hall CE, Murakoshi H, McNamara JO, Yasuda R. Rho GTPase complementation underlies BDNF-dependent homo- and heterosynaptic plasticity. Nature 2016; 538: 104–108.
Li N, Lee B, Liu RJ, Banasr M, Dwyer JM, Iwata M et al. mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science 2010; 329: 959–964.
Ota KT, Liu RJ, Voleti B, Maldonado-Aviles JG, Duric V, Iwata M et al. REDD1 is essential for stress-induced synaptic loss and depressive behavior. Nat Med 2014; 20: 531–535.
Dutheil S, Ota KT, Wohleb ES, Rasmussen K, Duman RS. High-fat diet induced anxiety and anhedonia: impact on brain homeostasis and inflammation. Neuropsychopharmacology 2016; 41: 1874–1887.
Burgdorf J, Zhang XL, Nicholson KL, Balster RL, Leander JD, Stanton PK et al. GLYX-13, a NMDA receptor glycine-site functional partial agonist, induces antidepressant-like effects without ketamine-like side effects. Neuropsychopharmacology 2013; 38: 729–742.
Sandhya VK, Raju R, Verma R, Advani J, Sharma R, Radhakrishnan A et al. A network map of BDNF/TRKB and BDNF/p75NTR signaling system. J Cell Commun Signal 2013; 7: 301–307.
Chen ZY, Jing D, Bath KG, Ieraci A, Khan T, Siao CJ et al. Genetic variant BDNF (Val66Met) polymorphism alters anxiety-related behavior. Science 2006; 314: 140–143.
Jourdi H, Hsu YT, Zhou M, Qin Q, Bi X, Baudry M. Positive AMPA receptor modulation rapidly stimulates BDNF release and increases dendritic mRNA translation. J Neurosci 2009; 29: 8688–8697.
Todd EL, Abernethy DR. Physiological pharmacokinetics and pharmacodynamics of (+/-)-verapamil in female rats. Biopharm Drug Dispos 1987; 8: 285–297.
Gao Q, Yao W, Wang J, Yang T, Liu C, Tao Y et al. Post-training activation of Rac1 in the basolateral amygdala is required for the formation of both short-term and long-term auditory fear memory. Front Mol Neurosci 2015; 8: 65.
Narita M, Takagi M, Aoki K, Kuzumaki N, Suzuki T. Implication of Rho-associated kinase in the elevation of extracellular dopamine levels and its related behaviors induced by methamphetamine in rats. J Neurochem 2003; 86: 273–282.
Sananbenesi F, Fischer A, Wang X, Schrick C, Neve R, Radulovic J et al. A hippocampal Cdk5 pathway regulates extinction of contextual fear. Nat Neurosci 2007; 10: 1012–1019.
Shirayama Y, Chen AC, Nakagawa S, Russell DS, Duman RS. Brain-derived neurotrophic factor produces antidepressant effects in behavioral models of depression. J Neurosci 2002; 22: 3251–3261.
Harward SC, Hedrick NG, Hall CE, Parra-Bueno P, Milner TA, Pan E et al. Autocrine BDNF-TrkB signalling within a single dendritic spine. Nature 2016; 538: 99–103.
Li N, Liu RJ, Dwyer JM, Banasr M, Lee B, Son H et al. Glutamate N-methyl-D-aspartate receptor antagonists rapidly reverse behavioral and synaptic deficits caused by chronic stress exposure. Biol Psychiatry 2011; 69: 754–761.
Fuchikami M, Thomas A, Liu R, Wohleb ES, Land BB, DiLeone RJ et al. Optogenetic stimulation of infralimbic PFC reproduces ketamine's rapid and sustained antidepressant actions. Proc Natl Acad Sci USA 2015; 112: 8106–8111.
Navarria A, Wohleb ES, Voleti B, Ota KT, Dutheil S, Lepack AE et al. Rapid antidepressant actions of scopolamine: Role of medial prefrontal cortex and M1-subtype muscarinic acetylcholine receptors. Neurobiol Dis 2015; 82: 254–261.
Egan MF, Kojima M, Callicott JH, Goldberg TE, Kolachana BS, Bertolino A et al. The BDNF val66met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell 2003; 112: 257–269.
Hosang GM, Shiles C, Tansey KE, McGuffin P, Uher R. Interaction between stress and the BDNF Val66Met polymorphism in depression: a systematic review and meta-analysis. BMC Med 2014; 12: 7.
Laje G, Lally N, Mathews D, Brutsche N, Chemerinski A, Akula N et al. Brain-derived neurotrophic factor Val66Met polymorphism and antidepressant efficacy of ketamine in depressed patients. Biol Psychiatry 2012; 72: e27–e28.
Luo L. Rho GTPases in neuronal morphogenesis. Nat Rev Neurosci 2000; 1: 173–180.
Nakayama AY, Harms MB, Luo L. Small GTPases Rac and Rho in the maintenance of dendritic spines and branches in hippocampal pyramidal neurons. J Neurosci 2000; 20: 5329–5338.
Tashiro A, Minden A, Yuste R. Regulation of dendritic spine morphology by the rho family of small GTPases: antagonistic roles of Rac and Rho. Cereb Cortex. 2000; 10: 927–938.
Kang MG, Guo Y, Huganir RL. AMPA receptor and GEF-H1/Lfc complex regulates dendritic spine development through RhoA signaling cascade. Proc Natl Acad Sci USA 2009; 106: 3549–3554.
Moghaddam B, Adams B, Verma A, Daly D. Activation of glutamatergic neurotransmission by ketamine: a novel step in the pathway from NMDA receptor blockade to dopaminergic and cognitive disruptions associated with the prefrontal cortex. J Neurosci 1997; 17: 2921–2927.
Zanos P, Moaddel R, Morris PJ, Georgiou P, Fischell J, Elmer GI et al. NMDAR inhibition-independent antidepressant actions of ketamine metabolites. Nature 2016; 533: 481–486.
Banerjee P, Donello J, Yoshitake T, Kehr J. Rapastinel (Glyx-13), a rapid acting antidepressant, does not increase extracellular levels of dopamine and glutamate in rat medial prefrontal cortex. American College of Neuropsychopharmacology, 55th Annual Meeting 2016 poster M54.
Acknowledgments
This study was supported by NIMH grants MH045481 (RSD), MH093897 (RSD), the state of Connecticut, Sumitomo Dainippon Pharma (TK), and a research grant from Allergan. RSD has consulted and/or received research support from Naurex, Allergan, Lilly, Forest, Johnson & Johnson, Taisho, Sunovion and Navitor.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Kato, T., Fogaça, M.V., Deyama, S. et al. BDNF release and signaling are required for the antidepressant actions of GLYX-13. Mol Psychiatry 23, 2007–2017 (2018). https://doi.org/10.1038/mp.2017.220
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/mp.2017.220
This article is cited by
-
Sigma-1 receptor activation mediates the sustained antidepressant effect of ketamine in mice via increasing BDNF levels
Acta Pharmacologica Sinica (2024)
-
M1 acetylcholine receptors in somatostatin interneurons contribute to GABAergic and glutamatergic plasticity in the mPFC and antidepressant-like responses
Neuropsychopharmacology (2023)
-
Intranasal Administration of Resolvin E1 Produces Antidepressant-Like Effects via BDNF/VEGF-mTORC1 Signaling in the Medial Prefrontal Cortex
Neurotherapeutics (2023)
-
IGF-1 release in the medial prefrontal cortex mediates the rapid and sustained antidepressant-like actions of ketamine
Translational Psychiatry (2022)
-
Cell-type specific modulation of NMDA receptors triggers antidepressant actions
Molecular Psychiatry (2021)