Abstract
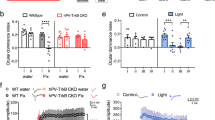
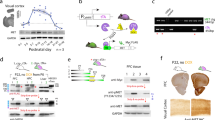
Accumulation of non-cell autonomous Otx2 homeoprotein in postnatal mouse visual cortex (V1) has been implicated in both the onset and closure of critical period (CP) plasticity. Here, we show that a genetic point mutation in the glycosaminoglycan recognition motif of Otx2 broadly delays the maturation of pivotal parvalbumin-positive (PV+) interneurons not only in V1 but also in the primary auditory (A1) and medial prefrontal cortex (mPFC). Consequently, not only visual, but also auditory plasticity is delayed, including the experience-dependent expansion of tonotopic maps in A1 and the acquisition of acoustic preferences in mPFC, which mitigates anxious behavior. In addition, Otx2 mis-localization leads to dynamic turnover of selected perineuronal net (PNN) components well beyond the normal CP in V1 and mPFC. These findings reveal widespread actions of Otx2 signaling in the postnatal cortex controlling the maturational trajectory across modalities. Disrupted PV+ network function and deficits in PNN integrity are implicated in a variety of psychiatric illnesses, suggesting a potential global role for Otx2 function in establishing mental health.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Hensch TK . Critical period plasticity in local cortical circuits. Nat Rev Neurosci 2005; 6: 877–888.
Hubel DH, Wiesel TN . The period of susceptibility to the physiological effects of unilateral eye closure in kittens. J Physiol 1970; 206: 419–436.
Wang BS, Sarnaik R, Cang J . Critical period plasticity matches binocular orientation preference in the visual cortex. Neuron 2010; 65: 246–256.
Sanke RF . Amblyopia. Am Fam Physician 1988; 37: 275–278.
Fagiolini M, Hensch TK . Inhibitory threshold for critical-period activation in primary visual cortex. Nature 2000; 404: 183–186.
Gogolla N, Leblanc JJ, Quast KB, Sudhof TC, Fagiolini M, Hensch TK . Common circuit defect of excitatory-inhibitory balance in mouse models of autism. J Neurodev Disord 2009; 1: 172–181.
Lewis DA, Curley AA, Glausier JR, Volk DW . Cortical parvalbumin interneurons and cognitive dysfunction in schizophrenia. Trends Neurosci 2012; 35: 57–67.
Kimoto S, Bazmi HH, Lewis DA . Lower expression of glutamic acid decarboxylase 67 in the prefrontal cortex in schizophrenia: contribution of altered regulation by Zif268. Am J Psychiatry 2014; 171: 969–978.
Uchida T, Furukawa T, Iwata S, Yanagawa Y, Fukuda A . Selective loss of parvalbumin-positive GABAergic interneurons in the cerebral cortex of maternally stressed Gad1-heterozygous mouse offspring. Transl Psychiatry 2014; 4: e371.
Failor S, Nguyen V, Darcy DP, Cang J, Wendland MF, Stryker MP et al. Neonatal cerebral hypoxia-ischemia impairs plasticity in rat visual cortex. J Neurosci 2010; 30: 81–92.
Do KQ, Cuenod M, Hensch TK . Targeting oxidative stress and aberrant critical period plasticity in the developmental trajectory to schizophrenia. Schizophrenia Bull 2015; 41: 835–846.
LeBlanc JJ, Fagiolini M . Autism: a "critical period" disorder? Neural Plast 2011; 2011: 921680.
Le Magueresse C, Monyer H . GABAergic interneurons shape the functional maturation of the cortex. Neuron 2013; 77: 388–405.
Sugiyama S, Di Nardo AA, Aizawa S, Matsuo I, Volovitch M, Prochiantz A et al. Experience-dependent transfer of Otx2 homeoprotein into the visual cortex activates postnatal plasticity. Cell 2008; 134: 508–520.
Beurdeley M, Spatazza J, Lee HH, Sugiyama S, Bernard C, Di Nardo AA et al. Otx2 binding to perineuronal nets persistently regulates plasticity in the mature visual cortex. J Neurosci 2012; 32: 9429–9437.
Spatazza J, Lee HH, Di Nardo AA, Tibaldi L, Joliot A, Hensch TK et al. Choroid-plexus-derived Otx2 homeoprotein constrains adult cortical plasticity. Cell Rep 2013; 3: 1815–1823.
Sabunciyan S, Yolken R, Ragan CM, Potash JB, Nimgaonkar VL, Dickerson F et al. Polymorphisms in the homeobox gene OTX2 may be a risk factor for bipolar disorder. Am J Med Genet B Neuropsychiatric Genet 2007; 144B: 1083–1086.
Pizzorusso T, Medini P, Berardi N, Chierzi S, Fawcett JW, Maffei L . Reactivation of ocular dominance plasticity in the adult visual cortex. Science 2002; 298: 1248–1251.
Miyata S, Komatsu Y, Yoshimura Y, Taya C, Kitagawa H . Persistent cortical plasticity by upregulation of chondroitin 6-sulfation. Nat Neurosci 2012; 15: S411–S412.
Bernard C, Prochiantz A . Otx2-PNN interaction to regulate cortical plasticity. Neural Plasticity 2016; 2016: 7931693.
Bernard C, Kim HT, Torero Ibad R, Lee EJ, Simonutti M, Picaud S et al. Graded Otx2 activities demonstrate dose-sensitive eye and retina phenotypes. Hum Mol Genet 2014; 23: 1742–1753.
Barkat TR, Polley DB, Hensch TK . A critical period for auditory thalamocortical connectivity. Nat Neurosci 2011; 14: 1189–1194.
Yang EJ, Lin EW, Hensch TK . Critical period for acoustic preference in mice. Proc Natl Acad Sci USA 2012; 109 (Suppl 2): 17213–17220.
Saxena A, Wagatsuma A, Noro Y, Kuji T, Asaka-Oba A, Watahiki A et al. Trehalose-enhanced isolation of neuronal sub-types from adult mouse brain. BioTechniques 2012; 52: 381–385.
Acampora D, Di Giovannantonio LG, Di Salvio M, Mancuso P, Simeone A . Selective inactivation of Otx2 mRNA isoforms reveals isoform-specific requirement for visceral endoderm anteriorization and head morphogenesis and highlights cell diversity in the visceral endoderm. Mech Dev 2009; 126: 882–897.
Acampora D, Mazan S, Lallemand Y, Avantaggiato V, Maury M, Simeone A et al. Forebrain and midbrain regions are deleted in Otx2-/- mutants due to a defective anterior neuroectoderm specification during gastrulation. Development 1995; 121: 3279–3290.
Nishida A, Furukawa A, Koike C, Tano Y, Aizawa S, Matsuo I et al. Otx2 homeobox gene controls retinal photoreceptor cell fate and pineal gland development. Nat Neurosci 2003; 6: 1255–1263.
Kang E, Durand S, LeBlanc JJ, Hensch TK, Chen C, Fagiolini M . Visual acuity development and plasticity in the absence of sensory experience. J Neurosci 2013; 33: 17789–17796.
Takesian AE, Hensch TK . Balancing plasticity/stability across brain development. Prog Brain Res 2013; 207: 3–34.
Garvert MM, Moutoussis M, Kurth-Nelson Z, Behrens TE, Dolan RJ . Learning-induced plasticity in medial prefrontal cortex predicts preference malleability. Neuron 2015; 85: 418–428.
Wang D, Fawcett J . The perineuronal net and the control of CNS plasticity. Cell Tissue Res 2012; 349: 147–160.
Carulli D, Pizzorusso T, Kwok JC, Putignano E, Poli A, Forostyak S et alAnimals lacking link protein have attenuated perineuronal nets and persistent plasticity Brain 2010; 133 (Pt 8): 2331–2347.
Morawski M, Filippov M, Tzinia A, Tsilibary E, Vargova L . ECM in brain aging and dementia. Prog Brain Res 2014; 214: 207–227.
Oohashi T, Edamatsu M, Bekku Y, Carulli D . The hyaluronan and proteoglycan link proteins: organizers of the brain extracellular matrix and key molecules for neuronal function and plasticity. Exp Neurol 2015; 274 (Pt B): 134–144.
Shen Y, Tenney AP, Busch SA, Horn KP, Cuascut FX, Liu K et al. PTPsigma is a receptor for chondroitin sulfate proteoglycan, an inhibitor of neural regeneration. Science 2009; 326: 592–596.
Dickendesher TL, Baldwin KT, Mironova YA, Koriyama Y, Raiker SJ, Askew KL et al. NgR1 and NgR3 are receptors for chondroitin sulfate proteoglycans. Nat Neurosci 2012; 15: 703–712.
Stephany CE, Chan LL, Parivash SN, Dorton HM, Piechowicz M, Qiu S et al. Plasticity of binocularity and visual acuity are differentially limited by nogo receptor. J Neurosci 2014; 34: 11631–11640.
Sato T, Kudo T, Ikehara Y, Ogawa H, Hirano T, Kiyohara K et al. Chondroitin sulfate N-acetylgalactosaminyltransferase 1 is necessary for normal endochondral ossification and aggrecan metabolism. J Biol Chem 2011; 286: 5803–5812.
Rossier J, Bernard A, Cabungcal JH, Perrenoud Q, Savoye A, Gallopin T et al. Cortical fast-spiking parvalbumin interneurons enwrapped in the perineuronal net express the metallopeptidases Adamts8, Adamts15 and Neprilysin. Mol Psychiatry 2015; 20: 154–161.
Valenzuela JC, Heise C, Franken G, Singh J, Schweitzer B, Seidenbecher CI et al. Hyaluronan-based extracellular matrix under conditions of homeostatic plasticity. Philos Trans R Soc Lond B Biol Sci 2014; 369: 20130606.
Zeisel A, Munoz-Manchado AB, Codeluppi S, Lonnerberg P, La Manno G, Jureus A et al. Brain structure. Cell types in the mouse cortex and hippocampus revealed by single-cell RNA-seq. Science 2015; 347: 1138–1142.
Prochiantz A, Di Nardo AA . Homeoprotein signaling in the developing and adult nervous system. Neuron 2015; 85: 911–925.
Kobayashi Y, Ye Z, Hensch TK . Clock genes control cortical critical period timing. Neuron 2015; 86: 264–275.
Gu Y, Huang S, Chang MC, Worley P, Kirkwood A, Quinlan EM . Obligatory role for the immediate early gene NARP in critical period plasticity. Neuron 2013; 79: 335–346.
Vo T, Carulli D, Ehlert EM, Kwok JC, Dick G, Mecollari V et al. The chemorepulsive axon guidance protein semaphorin3A is a constituent of perineuronal nets in the adult rodent brain. Mol Cell Neurosci 2013; 56: 186–200.
Giamanco KA, Matthews RT . Deconstructing the perineuronal net: cellular contributions and molecular composition of the neuronal extracellular matrix. Neuroscience 2012; 218: 367–384.
Tsien RY . Very long-term memories may be stored in the pattern of holes in the perineuronal net. Proc Natl Acad Sci USA 2013; 110: 12456–12461.
Werker JF, Hensch TK . Critical periods in speech perception: new directions. Annu Rev Psychol 2015; 66: 173–196.
Meredith RM, Dawitz J, Kramvis I . Sensitive time-windows for susceptibility in neurodevelopmental disorders. Trends Neurosci 2012; 35: 335–344.
Maeda N . Proteoglycans and neuronal migration in the cerebral cortex during development and disease. Front Neurosci 2015; 9: 98.
Gogolla N, Takesian AE, Feng G, Fagiolini M, Hensch TK . Sensory integration in mouse insular cortex reflects GABA circuit maturation. Neuron 2014; 83: 894–905.
Berretta S, Pantazopoulos H, Markota M, Brown C, Batzianouli ET . Losing the sugar coating: potential impact of perineuronal net abnormalities on interneurons in schizophrenia. Schizophr Res 2015; 167: 18–27.
Mauney SA, Athanas KM, Pantazopoulos H, Shaskan N, Passeri E, Berretta S et al. Developmental pattern of perineuronal nets in the human prefrontal cortex and their deficit in schizophrenia. Biol Psychiatry 2013; 74: 427–435.
Pantazopoulos H, Woo TU, Lim MP, Lange N, Berretta S . Extracellular matrix-glial abnormalities in the amygdala and entorhinal cortex of subjects diagnosed with schizophrenia. Arch Gen Psychiatry 2010; 67: 155–166.
Krishnan K, Wang BS, Lu J, Wang L, Maffei A, Cang J, Huang ZJ . MeCP2 regulates the timing of critical period plasticity that shapes functional connectivity in primary visual cortex. Proc Natl Acad Sci USA 2015; 112: E4782–E4791.
Belichenko PV, Hagberg B, Dahlstrom A . Morphological study of neocortical areas in Rett syndrome. Acta Neuropathol 1997; 93: 50–61.
Yizhar O, Fenno LE, Prigge M, Schneider F, Davidson TJ, O'Shea DJ et al. Neocortical excitation/inhibition balance in information processing and social dysfunction. Nature 2011; 477: 171–178.
Brown JA, Ramikie TS, Schmidt MJ, Baldi R, Garbett K, Everheart MG et al. Inhibition of parvalbumin-expressing interneurons results in complex behavioral changes. Mol Psychiatry 2015; 20: 1499–1507.
Akbarian S, Kim JJ, Potkin SG, Hagman JO, Tafazzoli A, Bunney WE Jr. et al. Gene expression for glutamic acid decarboxylase is reduced without loss of neurons in prefrontal cortex of schizophrenics. Arch Gen Psychiatry 1995; 52: 258–266.
Hashimoto T, Volk DW, Eggan SM, Mirnics K, Pierri JN, Sun Z et al. Gene expression deficits in a subclass of GABA neurons in the prefrontal cortex of subjects with schizophrenia. J Neurosci 2003; 23: 6315–6326.
Glausier JR, Fish KN, Lewis DA . Altered parvalbumin basket cell inputs in the dorsolateral prefrontal cortex of schizophrenia subjects. Mol Psychiatry 2014; 19: 30–36.
Turner CA, Thompson RC, Bunney WE, Schatzberg AF, Barchas JD, Myers RM et al. Altered choroid plexus gene expression in major depressive disorder. Front Hum Neurosci 2014; 8: 238.
Roybal K, Theobold D, Graham A, DiNieri JA, Russo SJ, Krishnan V et al. Mania-like behavior induced by disruption of CLOCK. Proc Natl Acad Scie USA 2007; 104: 6406–6411.
Rekaik H, Blaudin de The FX, Fuchs J, Massiani-Beaudoin O, Prochiantz A, Joshi RL . Engrailed homeoprotein protects mesencephalic dopaminergic neurons from oxidative stress. Cell Rep 2015; 13: 242–250.
Yinger OS, Gooding L . Music therapy and music medicine for children and adolescents. Child Adolescent Psychiatric Clin N Am 2014; 23: 535–553.
Acknowledgements
We thank M Nakamura for mouse maintenance; and support from NIH (1P50MH094271 and 1R01MH104488 to TKH), the Italian Association for Cancer Research (AIRC) (grant IG2013 N° 14152 to AS) and Fondation Bettencourt Schueller, ERC Advanced Grant HOMEOSIGN n° 339379 and ANR (ANR-11-BLAN-069467) (to AP). HHCL and ZY were further supported, respectively, by a post-doctoral fellowship from the Croucher Foundation (Hong Kong) and a Julius B Richmond predoctoral fellowship.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Molecular Psychiatry website
Rights and permissions
About this article
Cite this article
Lee, H., Bernard, C., Ye, Z. et al. Genetic Otx2 mis-localization delays critical period plasticity across brain regions. Mol Psychiatry 22, 680–688 (2017). https://doi.org/10.1038/mp.2017.1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/mp.2017.1
This article is cited by
-
Orthodenticle homeobox 2 is transported to lysosomes by nuclear budding vesicles
Nature Communications (2023)
-
Sensitive period-regulating genetic pathways and exposure to adversity shape risk for depression
Neuropsychopharmacology (2022)
-
Deconstructing the functional neuroanatomy of the choroid plexus: an ontogenetic perspective for studying neurodevelopmental and neuropsychiatric disorders
Molecular Psychiatry (2022)
-
Atypical perineuronal nets in the CA2 region interfere with social memory in a mouse model of social dysfunction
Molecular Psychiatry (2022)
-
Non-cell-autonomous OTX2 transcription factor regulates anxiety-related behavior in the mouse
Molecular Psychiatry (2021)