Abstract
Epidemiological studies report strong association between mood disorders and tobacco addiction. This high comorbidity requires adequate treatment but the underlying mechanisms are unknown. We demonstrate that nicotine exposure, independent of drug withdrawal effects, increases stress sensitivity, a major risk factor in mood disorders. Nicotine and stress concur to induce long-lasting cellular adaptations within the dopamine (DA) system. This interplay is underpinned by marked remodeling of nicotinic systems, causing increased ventral tegmental area (VTA) DA neurons’ activity and stress-related behaviors, such as social aversion. Blocking β2 or α7 nicotinic acetylcholine receptors (nAChRs) prevents, respectively, the development and the expression of social stress-induced neuroadaptations; conversely, facilitating α7 nAChRs activation specifically in the VTA promotes stress-induced cellular and behavioral maladaptations. Our work unravels a complex nicotine-stress bidirectional interplay and identifies α7 nAChRs as a promising therapeutic target for stress-related psychiatric disorders.
Similar content being viewed by others
Introduction
Psychiatric disorders, including drug addiction and depression, impose an alarming burden on society.1,2,3,4,5 Tobacco use is the leading cause of preventable death in developed countries. Yet, most smokers who wish to quit smoking encounter severe emotional and physical disturbances during drug withdrawal and only a small percentage succeed. These emotional manifestations include irritability, increased anxiety and depressed mood. The relation between nicotine and depressive symptoms is complex. Nicotine dependence increases more than threefold the vulnerability to develop depression.6,7 Conversely, depression symptoms are heavy determinants of smoking initiation, maintenance and cessation. For example, depression is associated with increased risk for smoking initiation8 and the appearance of early mental complications markedly increases the risk of later tobacco abuse.9,10 In addition, more severe drug withdrawal episodes have been reported in people with a history of depression, which impedes smoking cessation. This indicates a link between nicotine exposure and stress-related mood disorders.
These epidemiological and clinical data argue for a mutual maintenance pattern wherein each illness perpetuates the other. When two pathologies occur, simultaneously or sequentially, in the same person, they are described as comorbid. In addition to time correlation, comorbidity also implies interactions, as each disorder increases the course and prognosis of the other simultaneously.10 A major finding in psychopathology research is that comorbidity is the rule rather than the exception. However, why tobacco addiction and mood disorders often co-occur represents a major conundrum for neuroscientists. Importantly, as comorbid disorders often lack adequate treatment and are mostly associated with poor prognosis, this bidirectional relationship highlights the outdated strategy to treat each disease separately.
This intertwined, but at present unclear, relationship may arise from shared neuronal circuit adaptations. Despite their high heterogeneity with respect to genetic predisposing factors and behavioral manifestations of the symptoms, the above-mentioned pathologies involve influences over dopaminergic circuits. Neuronal nicotinic acetylcholine receptors (nAChRs) are key modulators of midbrain dopamine (DA) cell activity.11 The activation of nAChRs within the ventral tegmental area (VTA) by nicotine encodes tobacco’s primary reinforcing properties.5,12,13,14 Nicotine, in the absence of tobacco, is sufficient to sustain self-administration15 and produce reward in a conditioned place preference paradigm.16 Nicotine’s action requires numerous nAChRs subtypes segregated into high-affinity heteromeric nAChRs containing the β2 subunit (β2*) and low-affinity homomeric α7 nAChRs.17 They determine the sensitivity of the reward system to a minimum dose of nicotine necessary for VTA DA cell activation and thus nicotine reinforcement.18 VTA nAChRs subtypes are present on GABA and DA neurons, as well as on excitatory inputs, which results in a fine tuning of DA neuronal activity.11,12 Preclinical and clinical studies implicate both high- and low-affinity nAChRs in stress-induced pathologies. For example, changes in nAChRs availability have been observed in patients suffering from post-traumatic stress disorders or depression.19,20,21
Chronic social defeat stress (SD) parses the enduring behavioral abnormalities induced by stress and is believed to capture features of depressive-like states.22 SD triggers an increase in DA VTA neuronal activity23 that drives social avoidance. DA cells exhibit two distinct patterns of in vivo activity—a low-frequency tonic firing and a high-frequency phasic firing—with the possibility of switching amongst these modes. This mode switching is associated with reward-related behaviors and has been suggested to promote alertness in cases of threatening situations as observed after SD. Increased DA neuron activity is a prerequisite for the subsequent behavioral manifestations of stress outcomes.24,25,26,27 Indeed, pharmacological or optogenetic inhibition of VTA DA neurons promotes therapeutic-like effects as it reverses SD-induced social aversion and anhedonia.24,25
Thus, VTA DA neurons are at a crossroad of nicotine’s reinforcing effects and social stress-induced aversion. We hypothesized that nAChRs could be a pivotal molecular target, which would orchestrate the detrimental stress-nicotine interplay at the cellular and behavioral levels. To tackle this issue, we combined electrophysiology, pharmacology and radioligand binding approaches, as well as behavioral readouts in wild-type (WT) and nAChRs mutant mice. We demonstrate a bidirectional interplay between stress and nicotine exposure, which requires modulation of VTA DA neurons activity via α7 nAChRs.
Materials and methods
Further details regarding reagents, mice and procedures can be found in the Supplementary Information section.
Animals
Mice were housed under standard animal housing conditions.
SD stress paradigms
The chronic SD stress paradigm was performed as described in Barik et al. 24 Social interaction was performed 24 h after the last defeat (day 11). The substhreshold SD (SubSD) paradigm was adapted from Chaudhury et al. 25 Animals were individually introduced into a CD1 cage for 2 min of SD. The experimental and the CD1 mice were maintained separated through a partition for 20 min and then returned for 90 min to their home cage. The SD was repeated one time and mice were assessed in the social interaction test ~24 h later as described for the SD paradigm.
In vivo and in vitro electrophysiological recordings
Single-unit extracellular recordings of VTA DA cells were performed in anesthetized mice11 on day 11 for SD or on day 2 for SubSD. Nicotine injections were performed into the saphenous vein (intravenous). For in vitro patch-clamp experiment, mice were anesthetized for slice preparation on day 11 for SD or on day 2 for SubSD.
Pharmacological treatments
For chronic oral nicotine administration, mice were exposed to a gradually increasing dose of nicotine (50–200 μg ml−1) for a total of 19 days, submitted to SubSD and tested for social interaction without drug withdrawal. Methyllycaconitine (6 mg kg−1) and dihydro-β-erythroidine (1 mg kg−1) were administered intraperitoneally 15 min before each defeat session or before the target condition. PNU-120596 (1 mg kg−1, intraperitoneally) was administered 15 min before each defeat session of the SubSD paradigm (that is, two injections, Figure 4d), whereas nicotine (0.5 mg kg−1, free base, intraperitoneally) was administered right after each SD session. Mice were killed 24 h after for in vitro electrophysiological recordings.
Binding
WT mice were submitted to SD, tested for social interaction and brains from naive and stressed mice were snap frozen. For autoradiography, brains were processed as described in Metaxas et al. 28 For ligand binding, P2 fractions were prepared from dissected VTA samples to perform [3H]-Hemicholinium, [3H]-epibatidine and [125I]-α-Bungarotoxin binding.
Local VTA drug administration
Mice received an acute local injection of PNU-120596 (10 μM) and were then submitted to SubSD. After 20 min spent in the cage of the CD1 mouse with the partition in place, mice went directly through a second round of defeat. For local nicotine administration, mice were submitted to SubSD. Immediately after the first session of SD, nicotine (100 ng per site) was infused in the VTA, whereas mice were still facing the CD1 mouse through the partition preventing physical contacts. Nicotine-injected mice remained facing the CD1 for another 11 min before entering the second defeat session. After administration, mice were then returned to their home cage and tested for social interaction 24 h later.
Results
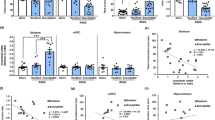
To determine whether SD impacts nicotinic systems in the VTA, mice were exposed to 10 days of SD (Figure 1a) and behavioral outcomes and cellular adaptations monitored. Expectedly, defeated (SD) WT mice displayed a marked social avoidance (Figure 1a) associated with an increased discharge frequency and bursting activity of VTA DA neurons (Figure 1b and Supplementary Figure S1a). In addition, using in vitro patch-clamp recordings, we observed that VTA DA neurons of defeated mice had an increase in AMPA.R/NMDA.R ratio (Figure 1c), which is likely to alter their output firing. These cellular adaptations may have relevance for nicotine actions on the DA system. To test this hypothesis, we measured DA neuron responses to intravenous nicotine administration in vivo. In naive mice (NoSD), a single intravenous injection of 30 μg kg−1 of nicotine,11,18 enhanced the firing and bursting activities of VTA DA cells (Figure 1d and Supplementary Figure S1b–g). In striking contrast, defeated mice exhibited a virtually abolished response to nicotine evidenced by a lack of significant variation from baseline of firing and bursting patterns of DA neurons (Figure 1d and Supplementary Figure S1b–g). This shows that social stress profoundly remodels VTA nicotinic systems. The ability of systemic nicotine to excite VTA DA neurons in naive conditions requires the activation of VTA nAChRs located on both GABA and DA neurons.11 Therefore, changes in nAChRs expression could explain a blunted nicotine response in SD conditions. Extensive brain mapping of α-bungarotoxin and epibatidine binding sites by autoradiography revealed no global alteration of α7 or β2* nAChRs numbers after SD, respectively (Supplementary Figures S2 and S3). Nonetheless, we next performed binding assays on VTA-dissected synaptic fractions from naive and SD mice, and we observed a modest, but significant increase in epibatidine, but not α-bungarotoxin, binding sites (Figure 1e). We hypothesized that the upregulation of epibatidine-sentitive β2* nAChR-binding sites is a result of prolonged exposure to the endogenous agonist acetycholine, and subsequent desensitization processes. We performed [3H]hemicholinium binding to quantify the density of choline transporters, which can be used as an index of endogenous cholinergic transmission. We observed a significant upregulation of choline transporter numbers following SD (Figure 1e). This result illustrates an enhanced cholinergic tone in the VTA after SD, as suggested in human depressed subjects.20
Behavioral and cellular interplay between social stress and nicotine responses in naive (no social defeat, NoSD) and defeated wild-type (WT) mice (social defeat, SD). (a) Schematic of the chronic SD paradigm (left). Decreased social interaction in SD mice compared to NoSD mice (right). (b) Firing rate (left panel) and percentage of spikes within bursts (%SWB; right panel) of ventral tegmental area (VTA) dopamine (DA) cells recorded in vivo. (Inset) sample in vivo firing activity recordings in DA neurons from NoSD and SD mice (51/38 mice). (c) SD increases AMPA.R/NMDA.R ratio assessed in VTA slices ex vivo. Inset: sample current traces recorded in DA neurons from NoSD and SD mice (4/4 mice). (d) The in vivo effects of acute intravenous nicotine (Nic) injection on DA firing frequency are reduced in SD mice when compared to NoSD WT mice (37/35 neurons; 27/25 mice). Inset left: Comparison of the mean±s.e.m. of increased variation from baseline in firing rate in NoSD and SD mice. (Inset right) firing activity sample trace of a DA cell recorded from a NoSD and SD mice before and after nicotine injection (dashed vertical red line). (e) Measurements of [3H]-epibatidine (left), [125I]-α-Bungarotoxin (middle) and [3H]-Hemicholinium (right) binding sites, performed on isolated VTA P2 fractions prepared from NoSD and SD mice (24/24 mice), suggest an upregulation of β2* nicotinic acetylcholine receptors (nAChRs) and an increased cholinergic tone in the VTA. Data are presented as mean±s.e.m., *P<0.05, **P<0.01 and ***P<0.01.
Hence, SD alters the spontaneous activity of DA neurons, their sensitivity to nicotine and increases the cholinergic tone in the VTA. If SD and nicotine29 induce similar alterations within the DA system, then nAChRs may be important mediators of these enduring changes and a promising therapeutic target. Brain α7 and β2* nAChRs constitute most of binding sites for nicotine, and these receptors can functionally compensate each other. Therefore, we first took a global approach and used α7β2 nAChRs double knockout mice that were exposed to SD to assess behavioral and cellular adaptations. Unlike defeated WT mice, defeated α7β2 nAChRs knockout mice did not display social aversion (Figure 2a), nor the expected increase in DA neurons activity (Figure 2b and Supplementary Figure S4a). As expected, acute intravenous nicotine failed to excite DA VTA neurons in both naive and defeated α7β2 nAChRs knockout mice (Figure 2c). In order to dissect the individual contribution of nAChRs subtypes to this effect, we next used pharmacological tools in WT mice. To address the role of α7 or β2* nAChRs in the development phase of SD-induced social avoidance and its underlying neuroadaptations during the 10 days of the SD paradigm, WT mice were chronically treated with systemic injections of saline or specific nAChRs antagonists (Figure 2d), 15 min before each daily defeat exposure. On day 11, the social interaction test was performed in a drug-free condition. Chronic treatment with dihydro-β-erythroidine, a selective β2* nAChRs antagonist, reversed the social avoidance behavior, whereas the chronic administration of methyllycaconitine did not (Figure 2d). To further examine the contribution of α7 or β2* nAChRs in the expression of social avoidance, naive and SD mice were only acutely challenged with saline or a specific nAChRs antagonist 15 min before the social interaction test (Figure 2e). In striking contrast with the development phase, acute administration of methyllycaconitine, that is devoid of locomotor effect (Supplementary Figure S5a), prevented SD-induced social aversion, whereas dihydro-β-erythroidine did not reverse stress effects but rather seemed to exacerbate it (Figure 2e). This indicates that α7 and β2* nAChRs have a dual pivotal role in the development and expression of stress-induced social aversion.
α7*nAChRs are required for social defeat (SD)-induced neurobehavioral adaptations. (a) Defeated (SD) α7β2 nicotinic acetylcholine receptors (nAChRs) knockout (KO) mice do not exhibit social avoidance. (b) Chronic stress fails to increase ventral tegmental area (VTA) dopamine (DA) neurons firing and bursting activity in α7β2 KO mice (7/12 mice). (c) Time course of the mean±s.e.m. variation from baseline of firing rate in α7β2 nAChRs KO mice control (NoSD) and following SD (7/12 mice). Bottom: comparison of the mean±s.e.m. of increased variation from baseline in firing frequency (left) and %SWB (right) in undefeated (NoSD) and defeated (SD) mice (7/12 mice). (d) Social interaction assessed in NoSD and SD mice that received during 10 days chronic treatment with an α7 nAChRs antagonist (methyllycaconitine, MLA) or a β2* nAChRs antagonist (dihydro-β-erythroidine, DHβE) before each daily SD episodes. (e) Social interaction assessed in NoSD and SD mice that received an acute injection of an α7 nAChRs antagonist (MLA) or a β2* nAChRs antagonist (DHβE) before the social interaction test. Data are presented as mean±s.e.m., *P<0.05, **P<0.01 and ***P<0.01.
Thus, the cellular dysregulations of the DA system by SD, which lead to behavioral maladaptations, require the biological substrate of nicotine. If stress hijacks the nicotinic system, then nicotine itself, via its action on VTA nAChRs, may be a risk factor for stress outcomes by altering the fine-tuning of DA cells activity. Therefore, nicotine exposure would be expected to increase the sensitivity of the DA system to a moderate social stress. To model this scenario, we treated mice with chronic nicotine in the drinking water, and then combined it with a subthreshold defeat (SubSD) paradigm (Figure 3a).25 We observed that SubSD triggered social aversion in mice chronically pretreated with nicotine, whereas it was ineffective in vehicle (saccharin)-pretreated mice (Figure 3b). Chronic nicotine treatment per se can induce enduring changes in VTA DA firing and bursting events (Figure 3c and Supplementary Figure S4b), and can also markedly impair DA neurons activation in response to a subsequent intravenous nicotine injection (Figure 3d). This prevents us from measuring any putative additive effects of SubSD at the cellular level in an attempt to understand the nicotine-stress interplay. To circumvent this issue, mice were challenged by acute, and not chronic, nicotine. WT mice were subjected to SubSD and immediately after received an acute systemic i.p. injection of saline or nicotine (0.5 mg kg−1). As depicted in Figure 3e, concomitant administration of acute nicotine with SubSD increased DA neurons excitability in vitro. Altogether, these results suggest that a short-lived stress episode primes VTA DA neurons, and that nicotine exposure exacerbates its magnitude, therefore constituting a risk factor for maintenance of cellular maladaptations at the basis of affective disorders.
Nicotine exacerbates behavioral and cellular outcomes of social stress in wild-type (WT) mice. (a) Schematic showing chronic nicotine (Nic) treatment followed with a subthreshold social defeat stress (SubSD) paradigm. (b) SubSD triggers social aversion in mice chronically treated with nicotine but not with saccharin (Sacch; right). (c) Firing rate and percentage of spikes within bursts (%SWB; right panel) of ventral tegmental area (VTA) dopamine (DA) cells recorded in vivo are increased in mice chronically treated with nicotine in the drinking water but not with saccharin (5/5 mice). (d) The in vivo effects of acute intravenous nicotine injection on DA firing frequency are reduced in chronically nicotine-treated mice (Sacch+Nic) when compared to saccharin-treated WT mice (17/10 neurons; 5/5 mice). (e) Excitability of VTA DA cells in mice that received systemic saline or nicotine (0.5 mg kg−1) with or without exposure to SubSD. Right: representative voltage traces from recorded neurons (Naive Veh: 3, Naive Nicotine: 4, SubSD Veh: 5, SubSD Nicotine 5 mice). Data are presented as mean±s.e.m., *P<0.05, **P<0.01 and ***P<0.001.
The potentiating effect of nicotine may reflect a gating influence over information flow within the DA system triggered by a moderate stress. We thus next tested whether this potentiation of nicotine effect can be recapitulated by activating only a specific nAChRs subtype. α7 nAChRs have often been involved in attentional functions and associative processes30 that are likely to be engaged during the expression of social aversion. Therefore, to probe for a specific involvement of α7* nAChRs in this process, we employed PNU-120596, an α7* nAChRs selective positive allosteric modulator.31,32 In WT mice primed by SubSD, acute systemic PNU-120596 produced cellular changes of VTA DA neurons (Figures 4a–c) that are comparable to neuroadaptations induced by SD. Indeed, VTA DA neurons of SubSD mice pre-treated with PNU-120596 exhibited increased in vitro excitability and AMPA.R/NMDA.R ratio (Figures 4a and b, respectively), as well as increased in vivo firing frequency and bursting activities (Figure 4c and Supplementary Figure S4c). As increases in DA neurons activity is a prerequisite for the manifestations of behavioral aspect of social stress,24,25 we would expect that PNU-120596 precipitate behavioral changes in SubSD mice. Indeed, systemic administration of PNU-120596 before SubSD, promoted a marked social aversion (Figure 4d). It is worth noting that PNU-120596 alone, at the dose used, did not induce changes in locomotor activity (Supplementary Figure S5b), nor did it show pro-depressant activity in the forced swim test (Supplementary Figure S6). In contrast, a systemic injection before SubSD of NS9283, a β2*nAChR-selective positive allosteric modulator,33 did not induce social avoidance behavior (Supplementary Figure S7). This demonstrates a specific role of α7* nAChRs in the expression of social avoidance induced by social stress.
α7* nAChRs activation promote stress sensitization. (a) Left panel: subthreshold social defeat (SubSD) combined with intraperitoneal (i.p.) drug injection paradigm. Right panel: PNU-120596, α7* selective agonist, treatment concomitant with SubSD causes a significant increase in ventral tegmental area (VTA) dopamine (DA) excitability measure ex vivo, (b) increase in AMPA/NMDA receptor ratio (Naive Veh: 3, Naive PNU: 4, SubSD Veh: 4, SubSD PNU 4 mice), and (c) in vivo firing (Hz) and bursting (% SWB) activity (NoSD Veh: 7, NoSD PNU: 4, SubSD Veh: 8, SubSD PNU 7 mice). (d) Social interaction after SubSD assessed in mice that received an acute injection of PNU-120596 (PNU). PNU administered concomitantly to SubSD promoted social aversion. Data are presented as mean±s.e.m., *P<0.05, **P<0.01 and ***P<0.01.
Our results suggest that nicotine exposure or activation of α7 nAChRs, can increase sensitivity to stress and promote long-lasting cellular and behavioral changes. However, due to the fact that we used systemic administration of drugs and the widespread expression of this nAChR subtype in the brain, it is not possible to relate these findings with a direct effect on the DA system. To ascertain the specific impact of nAChRs in the VTA as a minimal requirement to promote social avoidance, we used local infusion of drugs through implanted cannulas (Figure 5). Cannulated WT mice were given a single infusion of vehicle, PNU-120596 or nicotine within the VTA combined with a moderate acute stress. Concomitant administration of nicotine or PNU-120596 with SubSD triggered a marked social aversion (Figure 5), which therefore clearly implicates nAChRs in the VTA as mediators of stress outcomes.
Local activation of nAChRs in the VTA is a minimal requirement to promote social avoidance. WT mice were implanted with cannulas on the VTA and allow 1 week to recover. On the day of the experiment, mice were infused with PNU-120596 (1.2 ng per site) or nicotine (100 ng per site) and subjected to SubSD protocol. Concomitant intra-VTA PNU-120596 or nicotine and SubSD treatment resulted in significant social aversion. Data are presented as mean±s.e.m., *P<0.05 and **P<0.01.
Discussion
We have demonstrated that chronic social stress exposure modifies VTA nicotinic systems, a necessary condition for stress effects to occur and which requires a dual role of α7 and β2* nAChRs during the development and expression of the maladaptations. Conversely, we showed that nicotine potentiates stress outcomes, and that the effect of nicotine can be recapitulated by positively modulating α7 nAChRs function within the VTA. Overall, although several studies have described depression as a consequence of nicotine withdrawal, we here identify nicotine exposure per se as a risk factor for the development of stress-related disorders, independent of the withdrawal. This is a novel perspective that helps unravel the complex relationship between chronic stress exposure and nicotine addiction.
SD modifies nicotine-evoked responses
Mice exposed to SD develop marked avoidance of social contacts and exhibit several features that are reminiscent of human depression.34 The neuronal circuits that underpin stress effects are complex and far from being understood, but an increasing body of literature strongly supports a pivotal role of the DA reward system.23,24,26,34 SD triggers pronounced increases in VTA DA neuron spontaneous activity, which is causally associated with anhedonia and social aversion. Pre-exposure to stress is also well acknowledged to facilitate self-administration and precipitate relapse, and this is proposed to rely on changes of VTA DA neurons activity.3,35 Activation of DA neurons and subsequent release of DA in terminal fields are required for most drugs of abuse reinforcing and rewarding effects to take place.36,37 Consequently, dysregulation of DA neurons activity by stress affects subsequent drug responding. For example, acute restraint stress in rats increases ethanol self-administration and this correlates with an abolished ethanol-induced increase in DA neurons firing.38 Here we clearly show that chronic nicotine administration or chronic social stress blunts the ability of nicotine to activate VTA DA neurons. This could reflect a remodeling of inhibitory and excitatory local connectivity in the VTA. Stress and nicotine have been shown to decrease long-term potentiation of inhibitory synapses onto VTA DA neurons.39 In addition, rewarding and aversive stimuli trigger a strengthening of excitatory synapses onto DA neurons, marked by an increase in AMPA.R/NMDA.R ratio.40,41 Thus the net effect of abused drugs and stress is an enhanced activity of VTA DA neurons, potentially contributing to comorbidities.35 However, more than that, our result suggests a very specific interplay between stress and nicotine. Nicotine response is modified in stressed animals, because exposure to stress changes the primary substrate underlying nicotine response, that is, both cholinergic tone and nAChRs expression in the VTA. We revealed distinct key roles of β2* and α7* nAChRs during the development and the expression phases of stress-induced social aversion, respectively. From a potential therapeutic point of view, targeting α7 nAChRs may be more relevant to achieve stress relief.
Our work thus establishes a direct link between stress and nicotinic system, which could explain why stress-related diseases constitute a high-risk factor for nicotine addiction. We have previously shown that the sensitivity of VTA DA cells to nicotine-induced increase of activity determines the reinforcing dose of nicotine.18 The reduced response to nicotine exposure of VTA DA cells following SD may explain the high rates of tobacco consumption in stressed people or subjects suffering from mood disorders.
Nicotine exposure modifies stress susceptibility
Stress/addiction relationship is often viewed in a unidirectional way. Although it is well-known that stress increases tobacco consumption and maintenance, other evidence suggests that pre-exposure to nicotine can be an important factor to develop mood disorders.7 Our results strongly implicate modifications of the dopaminergic system. Nicotine exposure is known to regulate DA functions. This effect can be direct through nicotine action on nAChRs located within the dopaminergic system,12 or indirect through, for example, recruitment of neuroendocrine systems.42 Nicotine activates the hypothalamo-pituitary adrenal axis by primarily acting on neurons of the nucleus tractus solitarius projecting to the hypothalamus, which ultimately leads to the release of glucocorticoids hormones by the adrenal glands in both rodents and humans.43,44 Glucocorticoids can then strengthen excitatory transmission onto VTA DA neurons.41 We here demonstrate that nicotine by itself can increase stress susceptibility by amplifying acute stress effects. This is likely to be independent of hypothalamo-pituitary adrenal axis activation, as this effect can be replicated by local nicotine injection directly in the VTA.
SubSD is not sufficient to trigger social aversion in naive mice; however, it primes the dopaminergic system in an additive way to external challenges such as addictive substances. Concomitant nicotine and SubSD exposure trigger social aversion, in a similar manner that SD, by increasing DA cells activity. This rapid onset of the symptoms is much the same as the one observed with a selective optogenetic activation of DA neurons immediately after SubSD.25 Our results also demonstrated that either systemic or local VTA PNU-120596 exposure during SubSD is sufficient to trigger a marked social aversion. This differs from direct optogenetic activation of VTA DA neurons. Indeed, PNU-120596 is a positive allosteric modulator for α7*nAChRs, which per se does not directly increase VTA DA cells activity. The observed cellular and behavioral changes in the SubSD paradigm therefore reflect a change in endogenous acetylcholine release that is revealed by PNU-120596 administration. This is in accordance with the increase in [3H]hemicholinium binding, which suggests a marked change in cholinergic tone in the VTA after stress. This result suggests that cholinergic inputs to the VTA may constitute a signal for stress susceptibility, but more importantly, tightly links the susceptibility profile with nicotinic systems in the VTA. As α7 nAChRs facilitate stress impact on VTA DA neurons and are implicated in associative processes engaged in social avoidance, preventing their activation could counteract stress outcomes, and should be considered as a promising therapeutic target for depression.
Positive and negative salience in the VTA
In the SubSD paradigm, nicotine can promote social aversion. This gives the apparent paradox of a reinforcing substance that potentiates an aversive event. If midbrain DA neurons activation has long been associated with the processing of rewards, several studies have shown that they also have a role in signaling negative valence. Indeed, aversive stimuli including footpinch, noxious footshock or forced swim in cold water significantly affect DA neurons activity.40,45,46 This illustrates the complexity and multifunctional role of VTA DA neurons.47 SubSD per se does not affect excitability of VTA DA neurons, but overall this aversive stimulus is likely to initiate cellular processes affecting DA neurons homeostasis, which will consequently facilitate the action of further salient events such as nicotine intake. Hence, concomitant nicotine administration can strengthen an aversive stimulus by its action on DA neurons that have been previously primed by this mild stressor.
Positive and negative valence stimuli also induce similar changes in both VTA DA cells dynamics and synaptic plasticity. Glutamatergic inputs onto DA neurons are potentiated when animals are treated with abused substances such as cocaine or nicotine.48,49 In addition to a direct action on VTA nAChRs localized on DA and GABA neurons, nicotine, via presynaptic α7 nAChRs located on glutamatergic terminals, increases VTA glutamate concentration and can induce long-lasting potentiation of excitatory neurotransmission.50,51 In addition, a single in vivo administration of nicotine, via both β2* and α7 nAChRs, increases AMPA.R/NMDA.R ratio by enhancing GluA1-containing AMPA receptors at post-synaptic sites.49 Similar cellular outcomes are also produced by acute aversive stimuli via an enhancement in AMPA receptor function.40,52 Here we show that chronically stressed mice present a large increase in AMPA.R/NMDA.R ratio, however further studies will be necessary to determine if it reflects pre- or post-synaptic adaptations and address whether this modification share the same molecular mechanisms as those seen in acute treatment with abused substances or aversive stimuli.
These points emphasize the complexity of the role of VTA DA neurons in motivational processes and add evidence against a simplistic view of VTA DA cell function. Parallel synaptic and cellular changes seen after drug exposure and stress may provide a reason why acute stressors precipitate drug seeking. In this line, we show here that an acute stress event combined with nicotine or an α7 nAChRs positive modulator is sufficient to induce changes in firing activity and glutamatergic plasticity that resemble those of chronic stress or exposure to abused substances.
Nicotine and e-cigarettes
Our data also indicate that acute and chronic nicotine, which is delivered by tobacco smoke or e-cigarettes, could exacerbate the effects of social stress and thus increase the susceptibility to develop stress-related mood disorders. One of the major aims of public health policy is to reduce harm associate with smoking. E-cigarette is viewed as a safer way to provide smokers with nicotine, the addictive component, while sparing exposure to other chemical constituents of tobacco smoke that are responsible for a significant portion of the harm caused by smoking. Our work suggests that nicotine by itself can have important impact on mood dysregulation and should be manipulated with caution.
References
Bruijnzeel AW. Tobacco addiction and the dysregulation of brain stress systems. Neurosci Biobehav Rev 2012; 36: 1418–1441.
Cohen S, Lichtenstein E. Perceived stress, quitting smoking, and smoking relapse. Health Psychol 1990; 9: 466–478.
Piazza PV, Le Moal ML. Pathophysiological basis of vulnerability to drug abuse: role of an interaction between stress, glucocorticoids, and dopaminergic neurons. Annu Rev Pharmacol Toxicol 1996; 36: 359–378.
Siahpush M, Carlin JB. Financial stress, smoking cessation and relapse: results from a prospective study of an Australian national sample. Addiction 2006; 101: 121–127.
Picciotto MR, Mineur YS. Molecules and circuits involved in nicotine addiction: the many faces of smoking. Neuropharmacology 2013; 76: 545–553.
Klungsøyr O, Nygård JF, Sørensen T, Sandanger I. Cigarette smoking and incidence of first depressive episode: an 11-year, population-based follow-up study. Am J Epidemiol 2006; 163: 421–432.
Breslau N, Johnson EO. Predicting smoking cessation and major depression in nicotine-dependent smokers. Am J Public Health 2000; 90: 1122–1127.
Patton GC, Carlin JB, Coffey C, Wolfe R, Hibbert M, Bowes G. Depression, anxiety, and smoking initiation: a prospective study over 3 years. Am J Public Health 1998; 88: 1518–1522.
Volkow ND. The reality of comorbidity: depression and drug abuse. BPS 2004; 56: 714–717.
National Institute on Drug Abuse. Comorbidity: Addiction and Other Mental Illnesses. National Institute on Drug Abuse, Retrieved from https://www.drugabuse.gov/publications/research-reports/comorbidity-addiction-other-mental-illnesses on 2017, July 4.
Tolu S, Eddine R, Marti F, David V, Graupner M, Pons S et al. Co-activation of VTA DA and GABA neurons mediates nicotine reinforcement. Mol Psychiatry 2013; 18: 382–393.
Faure P, Tolu S, Valverde S, Naudé J. Role of nicotinic acetylcholine receptors in regulating dopamine neuron activity. Neuroscience 2014; 282C: 86–100.
Benowitz NL. Pharmacology of nicotine: addiction, smoking-induced disease, and therapeutics. Annu Rev Pharmacol Toxicol 2008; 49: 57–71.
Marti F, Arib O, Morel C, Dufresne V, Maskos U, Corringer P-J et al. Smoke extracts and nicotine, but not tobacco extracts, potentiate firing and burst activity of ventral tegmental area dopaminergic neurons in mice. Neuropsychopharmacology 2011; 36: 2244–2257.
Goodwin AK, Hiranita T, Paule MG. The reinforcing effects of nicotine in humans and nonhuman primates: a review of intravenous self-administration evidence and future directions for research. Nicotine Tob Res 2015; 17: 1297–1310.
Grabus SD, Martin BR, Brown SE, Damaj MI. Nicotine place preference in the mouse: influences of prior handling, dose and strain and attenuation by nicotinic receptor antagonists. Psychopharmacology 2006; 184: 456–463.
Zoli M, Pistillo F, Gotti C. Diversity of native nicotinic receptor subtypes in mammalian brain. Neuropharmacology 2014; 96: 302–311.
Morel C, Fattore L, Pons S, Hay YA, Marti F, Lambolez B et al. Nicotine consumption is regulated by a human polymorphism in dopamine neurons. Mol Psychiatry 2014; 19: 930–936.
Czermak C, Staley JK, Kasserman S, Bois F, Young T, Henry S et al. beta2 Nicotinic acetylcholine receptor availability in post-traumatic stress disorder. Int J Neuropsychopharmacol 2008; 11: 419–424.
Saricicek A, Esterlis I, Maloney KH, Mineur YS, Ruf BM, Muralidharan A et al. Persistent β2*-nicotinic acetylcholinergic receptor dysfunction in major depressive disorder. Am J Psychiatry 2012; 169: 851–859.
Mineur YS, Fote GM, Blakeman S, Cahuzac EL, Newbold SA, Picciotto MR. Multiple nicotinic acetylcholine receptor subtypes in the mouse amygdala regulate affective behaviors and response to social stress. Neuropsychopharmacology 2015; 41: 1579–1587.
Nestler EJ, Hyman SE. Animal models of neuropsychiatric disorders. Nat Neurosci 2010; 13: 1161–1169.
Cao J-L, Covington HE, Friedman AK, Wilkinson MB, Walsh JJ, Cooper DC et al. Mesolimbic dopamine neurons in the brain reward circuit mediate susceptibility to social defeat and antidepressant action. J Neurosci 2010; 30: 16453–16458.
Barik J, Marti F, Morel C, Fernandez SP, Lanteri C, Godeheu G et al. Chronic stress triggers social aversion via glucocorticoid receptor in dopaminoceptive neurons. Science 2013; 339: 332–335.
Chaudhury D, Walsh JJ, Friedman AK, Juarez B, Ku SM, Koo JW et al. Rapid regulation of depression-related behaviours by control of midbrain dopamine neurons. Nature 2013; 493: 532–536.
Friedman AK, Walsh JJ, Juarez B, Ku SM, Chaudhury D, Wang J et al. Enhancing depression mechanisms in midbrain dopamine neurons achieves homeostatic resilience. Science 2014; 344: 313–319.
Marinelli M, McCutcheon JE. Heterogeneity of dopamine neuron activity across traits and states. Neuroscience 2014; 282C: 176–197.
Metaxas A, Al-Hasani R, Farshim P, Tubby K, Berwick A, Ledent C et al. Genetic deletion of the adenosine A(2A) receptor prevents nicotine-induced upregulation of α7, but not α4β2* nicotinic acetylcholine receptor binding in the brain. Neuropharmacology 2013; 71: 228–236.
Besson M, Granon S, Mameli-Engvall M, Cloez-Tayarani I, Maubourguet N, Cormier A et al. Long-term effects of chronic nicotine exposure on brain nicotinic receptors. Proc Natl Acad Sci USA 2007; 104: 8155–8160.
Leiser SC, Bowlby MR, Comery TA, Dunlop J. A cog in cognition: how the alpha 7 nicotinic acetylcholine receptor is geared towards improving cognitive deficits. Pharmacol Ther 2009; 122: 302–311.
Hurst RS, Hurst RS, Hajós M, Hajós M, Raggenbass M, Raggenbass M et al. A novel positive allosteric modulator of the alpha7 neuronal nicotinic acetylcholine receptor: in vitro and in vivo characterization. J Neurosci 2005; 25: 4396–4405.
Livingstone PD, Dickinson JA, Srinivasan J, Kew JNC, Wonnacott S. Glutamate-dopamine crosstalk in the rat prefrontal cortex is modulated by Alpha7 nicotinic receptors and potentiated by PNU-120596. J Mol Neurosci 2010; 40: 172–176.
Mohler EG, Franklin SR, Rueter LE. Discriminative-stimulus effects of NS9283, a nicotinic α4β2* positive allosteric modulator, in nicotine-discriminating rats. Psychopharmacology 2014; 231: 67–74.
Krishnan V, Han M-H, Graham DL, Berton O, Renthal W, Russo SJ et al. Molecular adaptations underlying susceptibility and resistance to social defeat in brain reward regions. Cell 2007; 131: 391–404.
Dani JA, Harris RA. Nicotine addiction and comorbidity with alcohol abuse and mental illness. Nat Neurosci 2005; 8: 1465–1470.
Di Chiara G, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci USA 1988; 85: 5274–5278.
Koob GF, Volkow ND. Neurobiology of addiction: a neurocircuitry analysis. Lancet Psychiatry 2016; 3: 760–773.
Ostroumov A, Thomas AM, Kimmey BA, Karsch JS, Doyon WM, Dani JA. Stress increases ethanol self-administration via a shift toward excitatory GABA signaling in the ventral tegmental area. Neuron 2016; 92: 493–504.
Niehaus JL, Murali M, Kauer JA. Drugs of abuse and stress impair LTP at inhibitory synapses in the ventral tegmental area. Eur J Neurosci 2010; 32: 108–117.
Saal D, Dong Y, Bonci A, Malenka RC. Drugs of abuse and stress trigger a common synaptic adaptation in dopamine neurons. Neuron 2003; 37: 577–582.
Daftary SS, Panksepp J, Dong Y, Saal DB. Stress-induced, glucocorticoid-dependent strengthening of glutamatergic synaptic transmission in midbrain dopamine neurons. Neurosci Lett 2009; 452: 273–276.
Doyon WM, Thomas AM, Ostroumov A, Dong Y, Dani JA. Potential substrates for nicotine and alcohol interactions: a focus on the mesocorticolimbic dopamine system. Biochem Pharmacol 2013; 86: 1181–1193.
Mendelson JH, Sholar MB, Goletiani N, Siegel AJ, Mello NK. Effects of low- and high-nicotine cigarette smoking on mood states and the HPA axis in men. Neuropsychopharmacology 2005; 30: 1751–1763.
Armario A. Activation of the hypothalamic-pituitary-adrenal axis by addictive drugs: different pathways, common outcome. Trends Pharmacol Sci 2010; 31: 318–325.
Brischoux F, Chakraborty S, Brierley DI, Ungless MA. Phasic excitation of dopamine neurons in ventral VTA by noxious stimuli. Proc Natl Acad Sci USA 2009; 106: 4894–4899.
Ungless MA, Magill PJ, Bolam JP. Uniform inhibition of dopamine neurons in the ventral tegmental area by aversive stimuli. Science 2004; 303: 2040–2042.
Bromberg-Martin ES, Matsumoto M, Hikosaka O. Dopamine in motivational control: rewarding, aversive, and alerting. Neuron 2010; 68: 815–834.
Ungless MA, Whistler JL, Malenka RC, Bonci A. Single cocaine exposure in vivo induces long-term potentiation in dopamine neurons. Nature 2001; 411: 583–587.
Gao M, Jin Y, Yang K, Zhang D, Lukas RJ, Wu J. Mechanisms involved in systemic nicotine-induced glutamatergic synaptic plasticity on dopamine neurons in the ventral tegmental area. J Neurosci 2010; 30: 13814–13825.
Mansvelder HD, McGehee DS. Long-term potentiation of excitatory inputs to brain reward areas by nicotine. Neuron 2000; 27: 349–357.
Jones IW, Wonnacott S. Precise localization of alpha7 nicotinic acetylcholine receptors on glutamatergic axon terminals in the rat ventral tegmental area. J Neurosci 2004; 24: 11244–11252.
Lammel S, Ion DI, Roeper J, Malenka RC. Projection-specific modulation of dopamine neuron synapses by aversive and rewarding stimuli. Neuron 2011; 70: 855–862.
Acknowledgments
This work was supported by the CNRS UMR 8246, Institut Universitaire de France to JB, the Foundation for Medical Research (FRM, Equipe DEQ20130326488 to PF), ANR (ANR-13-JSV4-0004 to JB, ANR-16 Nicostress to PF and JB), the Bettencourt Schueller Foundation (Coup d'Elan 2012 to PF), the Labex Bio-Psy, the Institut National du Cancer to JB, UM and PF, the ‘SmokeFreeBrain Horizon 2020 grant (No681120)’ to AB, and the National Institute on Alcohol Abuse and Alcoholism (AA022445), the National Institute of Mental Health (MH112081), the NARSAD Independent Investigator Award to MHH. The laboratories of MM and PF are part of the École des Neurosciences de Paris Ile-de-France RTRA network. MM and PF are members of the Laboratory of Excellence, LabEx Bio-Psy. PF is a member of DHU Pepsy.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare no conflict of interest.
Electronic supplementary material
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article's Creative Commons license, unless indicated otherwise in the credit line; if thematerial is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Morel, C., Fernandez, S.P., Pantouli, F. et al. Nicotinic receptors mediate stress-nicotine detrimental interplay via dopamine cells’ activity. Mol Psychiatry 23, 1597–1605 (2018). https://doi.org/10.1038/mp.2017.145
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/mp.2017.145
This article is cited by
-
Nicotinic receptors promote susceptibility to social stress in female mice linked with neuroadaptations within VTA dopamine neurons
Neuropsychopharmacology (2022)
-
A non-canonical GABAergic pathway to the VTA promotes unconditioned freezing
Molecular Psychiatry (2022)
-
Midbrain projection to the basolateral amygdala encodes anxiety-like but not depression-like behaviors
Nature Communications (2022)
-
Chronic nicotine increases midbrain dopamine neuron activity and biases individual strategies towards reduced exploration in mice
Nature Communications (2021)
-
Alterations and adaptation of ventral tegmental area dopaminergic neurons in animal models of depression
Cell and Tissue Research (2019)