Abstract
Studies of infant siblings of older autistic probands, who are at elevated risk for autism, have demonstrated that the defining features of autism are not present in the first year of life but emerge late in the first and into the second year. A recent longitudinal neuroimaging study of high-risk siblings revealed a specific pattern of brain development in infants later diagnosed with autism, characterized by cortical surface area hyper-expansion in the first year followed by brain volume overgrowth in the second year that is associated with the emergence of autistic social deficits. Together with new observations from genetically defined autism risk alleles and rodent models, these findings suggest a conceptual framework for the early, post-natal development of autism. This framework postulates that an increase in the proliferation of neural progenitor cells and hyper-expansion of cortical surface area in the first year, occurring during a pre-symptomatic period characterized by disrupted sensorimotor and attentional experience, leads to altered experience-dependent neuronal development and decreased elimination of neuronal processes. This process is linked to brain volume overgrowth and disruption of the refinement of neural circuit connections and is associated with the emergence of autistic social deficits in the second year of life. A better understanding of the timing of developmental brain and behavior mechanisms in autism during infancy, a period which precedes the emergence of the defining features of this disorder, will likely have important implications for designing rational approaches to early intervention.
Similar content being viewed by others
Main
Studies employing the high familial risk for autism ‘infant sibling’ paradigm add substantially to our understanding of behavioral development in autism over the first few years of life.1 Chief among these findings is the overarching idea that the defining behaviors of autism spectrum disorder (ASD) are not evident in the first 6 months of life, but emerge during the latter part of the first and second years. The diagnostic features of autism, in high-risk (HR) infant siblings, appear to consolidate between 18 and 36 months of age. Relevant findings from prospective, longitudinal brain imaging studies of HR infant siblings are now becoming available. Findings from these studies, when combined with recent discoveries related to genetic and environmental risks for autism, enrich our understanding of possible neurodevelopmental mechanisms underlying very early brain and behavior development in autism. In this paper we propose a conceptual framework for the early, post-natal development of autism that integrates behavioral and brain imaging findings from HR, prospective infant sibling studies of autism. This developmental framework postulates that early increased proliferation of neuro-progenitor cells leads to a non-uniform, hyper-expansion of cortical surface area that links to early deficits in visual receptive/visual attention abilities which alter subsequent experience-dependent neuronal development more broadly in the brain. Cortical overgrowth results in a disruption in the refinement of selected neural circuit development and is associated with the emergence of social deficits in autism. Better understanding of the timing of developmental brain and behavior mechanisms in autism during infancy, a period which precedes the emergence of the defining features of this disorder, will likely have important implications for designing rational approaches to early intervention.
The Onset of the Defining Behaviors in the Syndrome of Autism
Genetic and environmental studies suggest that the pathophysiology of autism initiates pre-natally during mid-fetal development2, 3 and implicate excitatory neurons of the cerebral cortex.4, 5 However, there is now consensus in the field that the defining symptoms of autism emerge during the latter part of the first and second years of life. Several studies take a syndromic approach to assessing early manifestations of autism in infants employing the Autism Observation Scale for Infants (AOSI).1, 6 This direct behavioral assessment procedure revealed no differences at 6 months of age between HR infants who go on to be diagnosed with autism (HR-ASD), HR infants who are not later diagnosed as having autism (HR-negative) and low-risk (LR) infants.1, 7 Modest but significant differences on the AOSI were observed, however, in HR-ASD infants by 12 months of age in comparison to HR infants who do not go on to develop autism and low familial risk infants.1, 7, 8
Complementing the syndromic approach noted above, differences between select behavioral dimensions associated with autism were detected between groups at 12 months, but not earlier. For example, several studies reported that HR-ASD children differ from HR-negative and LR 12 month-olds in emerging language abilities,7, 9, 10, 11 aspects of social-cognition,12, 13 and repetitive behaviors.14, 15, 16 With few exceptions, the majority of studies examining the early emerging ASD behavioral phenotype are hindered by small sample sizes and methodological/conceptual variations in study design (Text Box 1).
Prodromal features of autism
Although observable behavior does not differentiate HR-ASD infants from their HR-negative and LR peers during the first six months of life, a number of preliminary studies highlight the importance of basic attentional operations and sensorimotor function in the early HR-ASD phenotype. Elison et al26 postulated that many of the observable signs of ASD in 12-month-olds (for example, inconsistently orienting to one’s name, diminished response to bids for joint attention, diminished spontaneous gaze to the face to extract social information, and inconsistently making eye contact), implicate behaviors associated with flexibly and efficiently allocating processing resources to salient or biologically relevant information in the environment. Indeed, several studies using eye tracking technology report that HR-ASD infants as young as 6 months of age allocate attentional resources to salient stimuli in a manner that differs from comparison groups in a disorder-specific fashion.26, 30, 36, 40 While the hypothesis stated above requires further exposition, visual orienting deficits have been identified in seven26 and 12-month-old later diagnosed with ASD.31, 41 Further, in LR infants, Elison, Paterson26 identified a specific association between visual orienting latencies and individual differences in organization of a white matter fiber bundle (the splenium of the corpus callosum) that projects through cortical areas important for visual and attentional processing,42 including posterior parietal and occipital cortices.43 This functional coupling was not observed in the HR-ASD group, suggesting atypical cortical function in this area that is critical for subsequent cognitive development.44, 45 The splenium also projects through the posterior hub of the default mode network, which has been characterized as early as the neonatal period,46 suggesting that this may be an important target for subsequent research on its role in the very early development of autism.
Differences in fine and gross motor skills observed at 6 months of age in HR-ASD children7 suggest that motor development in the first year of life may have a role in the development of autism.7, 32 The presence of increased motor stereotypes in HR-ASD infants14 at 12 months of age also points to abnormal development of motor systems, suggesting that processes that regulate diminishing gross motor rhythmic stereotypies between 6 and 12 months may be abnormal. Indeed sensorimotor systems that integrate and transform visual and auditory information into motor commands during the infant period play a prominent role in a recent computational model of ASD emergence.47 This model is augmented by preliminary evidence of abnormal neonatal brainstem function in graduates of the neonatal intensive care unit (NICU) who subsequently develop autism.48 Sensorimotor deficits and accompanying brain changes in associated regions are widely reported in autism beyond the infant period,49, 50, 51, 52, 53, 54, 55, 56, 57 as well as in first-degree relatives, supporting the sensorimotor domain as an endophenotype in autism.58
Taken together, this body of work indicates that the defining features of autism are not present at 6 months of age, but begin to emerge in the second year of life and appear to consolidate between 18 and 36 months. There is a striking paucity of data at 9–10 months of age, which may eventually alter what we currently know about the developmental emergence of defining characteristics of autism (but see refs. 29, 59, 60). To date, the majority of pre-symptomatic behavioral markers of autism, investigated in the first year of life, have been characterized with eye tracking technology. Attentional and sensorimotor functioning that supports basic information processing capacities, motor function, as well as flexibly and efficiently allocating processing resources to salient or biologically relevant information, represent critical targets for further investigation. This behavioral evidence also points to relevant neural systems implicated or associated with these behaviors, including the precuneus,61, 62 the posterior cingulate cortex,42 the intraparietal sulcus,63, 64 the corpus callosum26, 65, 66 and the cerebellum.67 Whether these structures play a foundational role in a cascade of downstream events, resulting in autism, is currently unclear.
Cortical surface area, brain overgrowth and the emergence of autistic behavior
One of the most consistent findings from studies of toddlers with autism has been a modest but significant increase in overall brain volume.68, 69, 70 Findings from a retrospective head circumference and prospective brain imaging study of two year olds with autism, followed up at age four years, indirectly suggested that brain enlargement was not present at birth but emerged at the end of the first and second year of life.71 Increased brain volume was associated with a stable increase in cortical surface area (but not cortical thickness) from two to four years of age.71 The presence of an early increase in surface area in association with brain enlargement, was also recently reported by Ohta and Nordahl.72
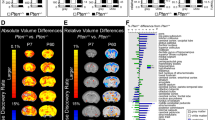
Hazlett, Gu73 prospectively assessed infants at high and low familial risk for autism, with brain imaging and cognitive-behavioral measures at 6, 12 and 24 months of age.73 HR subjects who were diagnosed with autism at 24 months of age were observed to have significant brain enlargement in comparison to both HR children who did not develop autism and those at LR with typical development. Brain enlargement was not present at 6 or 12 months of age, indicating that brain overgrowth at 24 months of age was the result of an accelerated rate of brain growth in the second year of life. HR infants later diagnosed with autism also showed an increased growth rate of cortical surface area from 6 to 12, but not 12 to 24 months of age in comparison to both the HR without autism and LR groups. No group differences were seen in cortical thickness across the 6-24 month interval. Exploratory analyses revealed that the greatest differences in surface area growth rate in HR-ASD infants were observed in the right middle occipital gyrus and left cuneus in visual cortex. This developmental perspective on individual-level change in brain growth rate, over a specific time period, as opposed to the finding of cross-sectional brain enlargement, has implications for our understanding of the number of autistic individuals potentially affected by this phenomenon and its relevance to understanding etiologic heterogeneity and pathogenesis in this disorder (see Text Box 2).
Surface area growth rate from 6 to 12 months in HR-ASD infants was significantly correlated with total brain volume growth rate from 12 to 24 months of age. Change in total brain volume from 12 to 24 (but not 6 to 12) months of age was associated with increased severity of autistic-like social deficits at 24 months on the Autism Diagnostic Observation Schedule (ADOS).74 Similar findings in severity of social deficits were observed at 12–24 but not 6–24 months of age, as measured on the Communication and Symbolic Behavior Scales.75 No significant relationship was observed between brain volume and repetitive behavior on the ADOS.
The pathophysiology of early autism
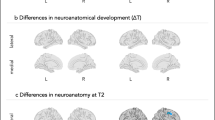
Taken together the findings described above suggest a temporal sequence of events in the developing brain of autistic individuals whereby hyper-expansion of surface area, beyond the non-uniform expansion of surface area noted in typically developing infants,82 co-occurs temporally with a prodromal period of behavioral (that is, motor-sensory and visual orienting) differences from 6 to 12 months of age. Subsequent brain overgrowth occurs in the second year of life, at a time when autistic social deficits are emerging and becoming more robust13 (Figure 1).
Early temporal relationships of brain and behavior in autism.
Hyper-expansion of surface area and brain volume
Early expansion of cortical surface area was initially thought to be due to the symmetrical, proliferation of neuronal progenitor cells, according to the radial unit hypothesis of Rakic.83 More recent refinements relevant to gyrencephalic mammals suggest that a specialized type of progenitor cell, exclusive to mammals with a folded cerebral cortex and referred to as the intermediate radial glia cell, is generated in the outer subventricular zone. Fan-like expansion of these radially migrating neurons is thought to drive tangential surface area growth and play a fundamental role in the ontogenetic and evolutionary expansion of the mammalian cerebral cortex.84, 85, 86, 87 The production of neurons through intermediate progenitor cells may precede development of the subventricular zone such that expanded numbers of intermediate progenitor cells that later lead to an increase in cortical surface area occurs before the onset of neurogenesis and without increasing the size of the ventricular zone.85, 88
Studies in mice have identified molecular contributors to progenitor cell proliferation and cortical surface area expansion such as β-catenin,89, 90 ARHGAP11B 91 and FGF signaling.92 Although it should be noted that mechanisms underlying surface area expansion in the gyrencephalic brains of humans may not fully correspond to those contributing to surface area expansion in the lissencephalic brains of rodent models; and thus rodent models of cortical surface area growth may not model all aspects of human neocortical brain development. Non-human primate models may prove especially important in this regard.
The genetic architecture of surface area has been described in humans,93 and differs from that underlying changes in cortical thickness.94, 95, 96 Genes involved with regulation of neuronal proliferation have also been linked to autism,97, 98 including a relationship to the social and stereotypical behaviors of autism in a mouse model dysregulating the β-catenin/Brn2/Tbr2 transcriptional cascade that results in expansion of basal neural progenitor cells.99 Indeed in a review of the genetics and post-mortem literature, Packer100 recently suggested that proliferation of neural progenitor cells potentially plays a central role in the pathogenesis of autism.
A recent large-scale, longitudinal, brain imaging study in human infants101 observed that early, post-natal surface area expansion in infancy is a principal driving factor in the growth of cortical volume at two years of age in the general population. Increased numbers of pre-frontal cortical neurons are reported in one post-mortem study102 of older children and adolescents with ASD, although differences in neuron number are not reported in others.103, 104 While proliferation of neural progenitor cells is not the only factor affecting cortical dimensions, it is considered a major early determinant. According to the “radial unit model” of Rakic83 cortical surface area is directly related to the number of radial units or so-called, “cortical mini-columns”. The report of an increased number of narrow, cortical mini-columns in the brains of autistic individuals is consistent with the above suggestion of an early increase in number of neurons in the brain in autism.105 However, these findings were not confirmed in a larger post-mortem study, where mini-columns in autistic subjects were found to be wider than controls, with more robust differences being found at younger ages.106
The early post-natal period in human brain development is also noted to be a time for sculpting of neuronal processes through pruning and apoptosis, largely beginning at the end of the first year.107 Decreased post-natal spine pruning resulting in an increased number of spines, was reported in a recent post-mortem study of ASD,108 suggesting this as a potential contributor to brain overgrowth and refinement of neural circuitry. Spine density was correlated with the hyper-activated mechanistic target of rapamycin (mTOR) and decreased neuronal autophagy. An autism mouse model of over-activated mTOR resulted in decreased postnatal spine pruning and autism-like social deficits that was corrected by the mTOR inhibitor rapamycin.108 The mTOR signaling pathway has been implicated in a number of genetically defined autistic syndromes such as PTEN-associated ASD (or Cowden Syndrome), a condition associated with macrocephaly and altered brain growth trajectory.94, 109 Kwon, Luikart110 demonstrated that inactivation of PTEN led to reduced social interaction and hyper-responsiveness to sensory stimuli in mice. PTEN mutants also have neuronal hypertrophy and macrocephaly. Conditional deletion of PTEN in lineages that generate interneurons increases the ratio of parvalbumin to somatostatin interneurons.111 Intriguingly, PTEN+/− mice show macrocephaly and an excess of cortical neurons at birth, and early brain overgrowth is driven by excess Wnt/β-catenin signaling.94
Other manipulations that alter Wnt/β-catenin pathway activity drive cortical overgrowth. The small molecule WNT pathway inhibitor XAV939 induced a transient amplification of intermediate progenitors, when injected into the developing neocortex of embryonic mice, followed by excessive production of excitatory pyramidal neurons in neocortical layers 2/3, and autism-like behaviors.81, 112 The excess of excitatory neurons impaired dendrite and spine development and affected the laminar distribution of interneurons. Dysregulation of neuronal development consequently altered excitatory and inhibitory synaptic connections and strength, causing an imbalance between synaptic excitation and inhibition, consistent with the Excitatory/Inhibitory Imbalance Theory of autism.113, 114 Mice exhibited autistic-like behavioral changes in social interaction and repetitive behaviors, suggesting a mechanistic link between early over-proliferation of cortical neurons and development of neuronal processes that impacts functional circuitry and produces an autistic-like behavior profile. Likewise, in utero exposure to the anti-epileptic valproic acid, known to increase risk for autism in humans2 and a well-recognized animal model of autistic behaviors,115 leads to expansion of upper layer cortical neurons.116
Consistent with the hypothesis that brain enlargement in autism is due to altered progenitor cell proliferation leading to network dysregulation are the recent findings by Marchetto, Belinson117 examining induced pluripotent stem cells, neural progenitor cells and neurons from individuals with ASD who demonstrated early brain overgrowth on MRI. ASD-derived neurons showed abnormal neurogenesis and reduced synaptogenesis, leading to functional deficits in neuronal networks. This process was reversed with administration of insulin-like growth factor 1 (IGF-1) suggesting a potential underlying cellular mechanism for targeted intervention.
Converging evidence from studies of genetically defined autistic conditions
Etiological heterogeneity is ubiquitous in autism. The framework put forward in this paper derives largely from findings in HR infant sibling studies and is therefore defined by the subset of infants who presumably have ‘idiopathic’ autism and a high familial liability for this condition. While etiologies differ in autistic individuals, there is likely to be more convergence at a mechanistic level with at least some other subgroups of autistic individuals.
Several genetically defined autism syndromes (for example, 16p11 deletion, PTEN, and Chd8 mutations) are associated with increased brain volume and macrocephaly 118.110, 119 Qureshi, Mueller119 reported brain volume enlargement and expanded cortical surface area (but not cortical thickness) in individuals with deletion 16p11, a copy number variant associated with autism;120 whereas brain volume and surface area were decreased in those with duplication 16p11. Examination of a murine model of human 16p11 deletion revealed enhanced neuro-progenitor cell proliferation and premature cell-cycle exit.121 Wang and Lin122 examined Chd8 in induced pluripotent stem cells (iPSCs) to better mimic the loss-of-function status that would exist in the developing human embryo prior to neuronal differentiation. Genome-wide association studies by these investigators identified seven of the twelve genes associated with human brain volume or head size were dysregulated in Chd8 (+/−) neural progenitors and neurons, as well as finding differential expression of β-catenin signaling. Transcriptomic profiling revealed that Chd8 regulates multiple genes implicated in ASD as well as brain volume. Finally, Chd8-deficient mice showed slight activation of Wnt/β-catenin signaling embryonically, an enlarged brain and autism-like phenotypes,123 while Chd8 knockdown early in cortical development (E13) impaired progenitor proliferation and led to depletion of the progenitor pool, which would ultimately reduce neuron number.124 Future studies will be needed to evaluate the cellular basis for brain overgrowth in Chd8 mutant mice, and to evaluate developmental stage and cell context-dependent effects of WNT signaling on brain overgrowth.125
Considered together these studies suggest a potential cellular mechanism underlying brain overgrowth in autism – a primary increase in intermediate neural progenitor cells, possibly driven by abnormal Wnt pathway activation, leading to an increase in cortical neuron number, an increase in brain volume and an imbalance in neural connectivity.126 There is increasing recognition that some high confidence autism-linked mutations, like DYRK1, cause microcephaly.127 Consistent with the clinical and etiologic heterogeneity known to be present in the behaviorally defined syndrome of autism, and consistent with the above referenced study showing decreased cortical surface area and brain volume in duplication 16p11, it should be emphasized that a primary increase in intermediate neural progenitor cells, may not be an underlying mechanism seen in autistic individuals who show no signs of macrocephaly or increased rate of brain growth and instead are microcephalic.119
A Conceptual framework for early brain and behavior development in autism
Integrating brain and behavior
In typically developing infants, expansion of cortical surface area in the first year of life is most robust in visual cortex.82 Surface area expansion in this same region is even more robust in infants later diagnosed with autism.73 Hyper-expansion of surface area in visual cortex during the latter part of the first year may underlie the sensorimotor and visual orienting deficits observed during this period in infants later diagnosed with autism.26 These sensory/ attentional deficits may alter experience-dependent neuronal development resulting in decreased sculpting of neuronal processes and brain overgrowth and disruption of selected brain connections, which in turn leads to the development of autistic-like social deficits (see Figure 2). Alternatively, a primary increase in intermediate neural progenitor cells leading to an increase in neuron number, may directly result in a downstream increase in brain volume. Increases in neuron number may reduce efficient sensorimotor and attentional functioning observed in the first year of life. As suggested by the work of Fang et al81 and Marchetto et al,117 abnormal brain overgrowth could disrupt synapse function and contribute to the emergence of autistic-like social and cognitive deficits in the early post-natal period.7
Cascading brain and behavior changes in the development in autism.
Other brain characteristics in autism during infancy
The above conceptual framework describes a sequence of events in the pathogenesis of brain overgrowth and the emergence of social deficits in autism. While we have built a story around two key findings newly emerging in the HR infant sibling brain imaging literature, other early brain differences have been identified in HR infant siblings in infancy. In HR-ASD infants generalized (multiple fiber tracts throughout the brain) abnormalities in white matter organizational structure have been reported65, 128 as early as 6 months of age. Aberrant white matter organizational structure has been observed by 6 months of age in the genu of the corpus callosum65 and white matter organization in the genu and cerebellar peduncles is significantly associated with sensory hypo- and hyper-responsivity at 24 months of age.129 In addition, other reports have demonstrated that increased extra-axial cerebrospinal fluid volume (EA-CSF) is present by 6 months of age in HR infants who are later diagnosed with autism at 24 months of age.38, 39 Enlargement of the extra-axial fluid compartment suggests diminished uptake and flow of cerebrospinal fluid (CSF) with accumulation of brain metabolites (for example, β-amyloid and pro-inflamatory cytokines).130, 131 Increased extra-axial fluid volume in infancy has been linked to motor deficits39, 132, 133, 134 as well as over-proliferation of neuronal progenitor cells.135 This latter observation led Lehtinen, Zappaterra135 to suggest that CSF composition may have critical relevance to the pathogenesis of neurodevelopmental disorders and is consistent with the findings by Hazlett, Gu 73 suggesting early post-natal hyper-proliferation in autism.
In a small study, disrupted connectivity measured via quasi-resting electroencephalography (EEG) differentiated HR in 14-month-old infants later diagnosed with autism from a HR-negative group and LR controls.136 Interestingly, oscillations in the alpha wave are thought to reflect processes related to visual and/or attentional engagement. While source localization of scalp recorded electrophysiological activity remains an enduring challenge, EEG and ERP studies have the potential to elucidate processing differences in the early autism phenotype. Indeed, there is a growing body of work comparing HR and LR infants (Text Box 1), yet there are very few EEG/ERP studies that directly compare processing between outcome groups (HR-ASD, HR-negative and LR) in the first year of life.29, 137
The potential for time-dependent pathophysiological mechanisms to instantiate cascading neurobiological processes is consistent with the theoretical framework for neurobehavioral development, ‘Interactive Specialization’.138 This model presumes that instantiating pathophysiology is transient99 and/or likely to be obfuscated by subsequent cascading neurobiological effects or disease progression. ASD imaging studies have revealed decreasing cortical thickness but no change in surface area, in older autistic individuals,139, 140 suggesting a protracted period of dynamic, neurobiological development in autism, throughout childhood and into the adult years. The impact of the intersection of life experiences and accumulating effects of autistic social, cognitive and sensory impairments must be considered as perhaps having an additional, intermediate and long-term role in experience-dependent brain development at later ages.141
Regional surface area hyper-expansion, sensory deficits and excitatory/inhibitory imbalance
Hazlett, Gu73 observed the most robust and significant surface area hyper-expansion in HR-ASD infants to occur in right middle occipital gyrus (Brodman Area 18) and the left cuneus (Brodman Area 17), both involved with visual processing. Increased surface area in primary visual cortex has been linked to increased GABA concentration in medial occipital cortex.142 Mariani et al143 conducted whole genome sequencing on IPSC lines derived from macrocephalic individuals with ASD and detected significantly perturbed transcriptomic signatures involving regulation of cell proliferation and neuronal differentiation that appeared to be due to a decrease in cell-cycle length. Further analyses suggested a greater proportion of GABAergic neurons in cortical organoids from ASD-derived neurons. These authors concluded that there is an early increase in proliferation of GABAergic neuronal progenitor cells in ASD-derived organoids that give rise to an increased proportion of mature GABAergic interneurons.
Experience during critical periods in early postnatal life refines cortical circuitry. Critical periods are regulated by the balance of excitatory and inhibitory (E/I) neurotransmission in the brain during development.144 Rubenstein and Merzenich113 first proposed an E/I imbalance in autism. More recently, it has been proposed that alteration of the expression and/or timing of critical period circuit refinement in primary sensory brain areas may significantly contribute to autistic phenotypes, including cognitive and behavioral impairments.145 The timing and location of surface area changes in the early development of autism suggests that regional hyper-proliferation of neural progenitor cells leading to brain overgrowth and disruption of vulnerable neural circuits may play a fundamental role in the development of autism. Consistent with the role of sensory experiences in the development of autism,146 Angelman syndrome model mice deficient in Ube3a, were observed to have profound impairments in neocortical plasticity in visual cortex, however plasticity was preserved during dark rearing. Altered early sensory experience may indeed be a primary deficit in autism resulting in downstream changes that we more traditionally associate with the defining features of autism e.g., social deficits. Understanding the impact of aberrant sensory function and experience in the first year of life may provide insights relevant to early intervention. Indeed a small, pilot study employing the Early Start Denver behavior intervention model, an approach that is heavily based on reinforcing and synchronizing selected sensory inputs in infants with autism as well as those at risk for autism, suggested that this type of very early intervention may be particularly beneficial.147 Wass, Porayska-Pomsta148 employed a gaze-contingent eye tracking paradigm and demonstrated ability to train attentional control in 11-month-old infants. Given the findings by Elison, Paterson26 and others regarding altered early visual orienting in infants who later demonstrate the defining features of autism, this approach offers a plausible avenue for targeted prodromal intervention in autism.
Caveats and future directions
Findings regarding accelerated growth rate of cortical surface area and brain volume require replication. As discussed in Text Box 2, the concept of increased brain growth rate within individuals has implications for understanding the etiologic heterogeneity in autism and specifically the number of affected individuals for whom this mechanism is relevant. Future studies will also need to examine the specificity of increased brain growth rate to autism versus other neurodevelopmental disorders both in etiologically (for example, Fragile X Syndrome) and phenotypically (for example, language and learning disorders) defined conditions. Large-scale prospective studies will provide additional insights into risk and protective factors in the cascading brain and behavior changes that result in autism in 2–3 years old infants, including the effects of genes, exposure to environmental toxins and experience. Finally, links to mechanisms require further exploration e.g., examination of the relationship between findings from induced pluripotent stem cells and cellular development to more refined phenotypes such as hyper-expansion of cortical surface area in the first year of life.
In summary, a conceptual framework for the early, post-natal development of autism is proposed that integrates behavioral and brain imaging findings from HR, prospective infant sibling studies of autism. These findings are consistent with brain and behavior studies about autism at older ages, post-mortem studies, as well as findings from genetically defined autistic syndromes and corresponding mouse models. This developmental framework postulates that early increased proliferation of neuro-progenitor cells leads to a non-uniform, hyper-expansion of cortical surface area that leads to early deficits in visual receptive/visual attention abilities that alter subsequent experience-dependent neuronal development more broadly in the brain. Cortical overgrowth results in a disruption in the refinement of selected neural circuit development and is associated with the emergence of social deficits in autism. Thus autism appears to primarily be a disorder of early sensory-motor-attention impairment, associated with hyper-proliferation of neuronal progenitor cells; and secondarily resulting in the defining features of autism and, in particular, social deficits. Better understanding of the timing of developmental brain and behavior mechanisms in autism during infancy, a period which precedes the emergence of the defining features of this disorder, will likely have important implications for designing rational approaches to early intervention.
References
Zwaigenbaum L, Bryson S, Rogers T, Roberts W, Brian J, Szatmari P . Behavioral manifestations of autism in the first year of life. Int J Dev Neurosci 2005; 23: 143–152.
Christensen J, Gronborg TK, Sorensen MJ, Schendel D, Parner ET, Pedersen LH et al. Prenatal valproate exposure and risk of autism spectrum disorders and childhood autism. JAMA 2013; 309: 1696–1703.
Fujiwara T, Morisaki N, Honda Y, Sampei M, Tani Y . Chemicals, nutrition, and autism spectrum disorder: a mini-review. Front Neurosci 2016; 10: 174.
Willsey AJ, Sanders SJ, Li M, Dong S, Tebbenkamp AT, Muhle RA et al. Coexpression networks implicate human midfetal deep cortical projection neurons in the pathogenesis of autism. Cell 2013; 155: 997–1007.
Parikshak NN, Luo R, Zhang A, Won H, Lowe JK, Chandran V et al. Integrative functional genomic analyses implicate specific molecular pathways and circuits in autism. Cell 2013; 155: 1008–1021.
Bryson SE, Zwaigenbaum L, McDermott C, Rombough V, Brian J . The Autism Observation Scale for Infants: scale development and reliability data. J Autism Dev Disord 2008; 38: 731–738.
Estes A, Zwaigenbaum L, Gu H St, John T, Paterson S, Elison JT et al. Behavioral, cognitive, and adaptive development in infants with autism spectrum disorder in the first 2 years of life. J Neurodev Disord 2015; 7: 24.
Gammer I, Bedford R, Elsabbagh M, Garwood H, Pasco G, Tucker L . Behavioural markers for autism in infancy: scores on the Autism Observational Scale for Infants in a prospective study of at-risk siblings. Infant Behav Dev 2015; 38.
Ozonoff S, Young GS, Belding A, Hill M, Hill A, Hutman T . The broader autism phenotype in infancy: when does it emerge? J Am Acad Child Adolesc Psychiatry 2014; 53: 398–407.
Mitchell S, Brian J, Zwaigenbaum L, Roberts W, Szatmari P, Smith I . Early language and communication development of infants later diagnosed with autism spectrum disorder. J Develop Behavior Pediatr 2006; 27: S69–S78.
Lazenby DC, Sideridis GD, Huntington N, Prante M, Dale PS, Curtin S et al. Language Differences at 12 Months in Infants Who Develop Autism Spectrum Disorder. J Autism Dev Disord 2016; 46: 899–909.
Landa RJ, Holman KC, Garrett-Mayer E . Social and communication development in toddlers with early and later diagnosis of autism spectrum disorders. Arch Gen Psychiatry 2007; 64: 853–864.
Ozonoff S, Iosif A, Baguio F, Cook IC, Hill MM, Hutman T . A prospective study of the emergence of early behavioral signs of autism. J Am Acad Child Adolesc Psychiatry. 2010; 49: 256–266.
Elison JT, Wolff JJ, Reznick JS, Botteron KN, Estes AM, Gu H . Repetitive behavior in 12-month-olds later classified with autism spectrum disorder. J Am Acad Child Adolesc Psychiatry 2014; 53: 1216–1224.
Ozonoff S, Heung K, Byrd R, Hansen R, Hertz-Picciotto I . The onset of autism: patterns of symptom emergence in the first years of life. Autism Res 2008; 1: 320–328.
Wolff JJ, Botteron KN, Dager SR, Elison JT, Estes AM, Gu H et al. Longitudinal patterns of repetitive behavior in toddlers with autism. J Child Psychol Psychiatry 2014; 55: 945–953.
Johnson MH, Gliga T, Jones E, Charman T . Annual research review: Infant development, autism, and ADHD--early pathways to emerging disorders. J Child Psychol Psychiatry 2015; 56: 228–247.
Szatmari P, Chawarska K, Dawson G, Georgiades S, Landa R, Lord C et al. Prospective longitudinal studies of infant siblings of children with autism: lessons learned and future directions. J Am Acad Child Adolesc Psychiatry 2016; 55: 179–187.
Klin A, Shultz S, Jones W . Social visual engagement in infants and toddlers with autism: early developmental transitions and a model of pathogenesis. Neurosci Biobehav Rev 2015; 50: 189–203.
Messinger DS, Young GS, Webb SJ, Ozonoff S, Bryson SE, Carter A et al. Early sex differences are not autism-specific: A Baby Siblings Research Consortium (BSRC) study. Mol Autism 2015; 6: 32.
Ozonoff S, Young GS, Carter A, Messinger D, Yirmiya N, Zwaigenbaum L et al. Recurrence risk for autism spectrum disorders: a Baby Siblings Research Consortium study. Pediatrics 2011; 128: e488–e495.
Sandin S, Lichtenstein P, Kuja-Halkola R, Larsson H, Hultman CM, Reichenberg A . The familial risk of autism. JAMA 2014; 311: 1770–1777.
Charman T, Young GS, Brian J, Carter A, Carver LJ, Chawarska K et al. Non-ASD outcomes at 36 months in siblings at familial risk for autism spectrum disorder (ASD): A baby siblings research consortium (BSRC) study. Autism Res 2016; 10: 169–178.
Messinger D, Young GS, Ozonoff S, Dobkins K, Carter A, Zwaigenbaum L et al. Beyond autism: a baby siblings research consortium study of high-risk children at three years of age. J Am Acad Child Adolesc Psychiatry 2013; 52: 300–308, e1.
Ozonoff S, Young GS, Belding A, Hill M, Hill A, Hutman T et al. The broader autism phenotype in infancy: when does it emerge? J Am Acad Child Adolesc Psychiatry 2014; 53: 398–407, e2.
Elison JT, Paterson SJ, Wolff JJ, Reznick JS, Sasson NJ, Gu H et al. White matter microstructure and atypical visual orienting in 7-month-olds at risk for autism. Am J Psychiatry 2013; 170: 899–908.
Wass SV, Jones EJ, Gliga T, Smith TJ, Charman T, Johnson MH . Shorter spontaneous fixation durations in infants with later emerging autism. Sci Rep 2015; 5: 8284.
Finch KH, Seery AM, Talbott MR, Nelson CA, Tager-Flusberg H . Lateralization of ERPs to speech and handedness in the early development of Autism Spectrum Disorder. J Neurodev Disord 2017; 9: 4.
Elsabbagh M, Mercure E, Hudry K, Chandler S, Pasco G, Charman T et al. Infant neural sensitivity to dynamic eye gaze is associated with later emerging autism. Curr Biol 2012; 22: 338–342.
Chawarska K, Macari S, Shic F . Decreased spontaneous attention to social scenes in 6-month-old infants later diagnosed with autism spectrum disorders. Biol Psychiatry 2013; 74: 195–203.
Elsabbagh M, Fernandes J, Jane Webb S, Dawson G, Charman T, Johnson MH . Disengagement of visual attention in infancy is associated with emerging autism in toddlerhood. Biol Psychiatry 2013; 74: 189–194.
Flanagan JE, Landa R, Bhat A, Bauman M . Head lag in infants at risk for autism: a preliminary study. Am J Occup Ther. 2012; 66: 577–585.
Jones W, Klin A . Attention to eyes is present but in decline in 2-6-month-old infants later diagnosed with autism. Nature 2013; 504: 427–431.
Keehn B, Vogel-Farley V, Tager-Flusberg H, Nelson CA . Atypical hemispheric specialization for faces in infants at risk for autism spectrum disorder. Autism Res 2015; 8: 187–198.
Paul R, Fuerst Y, Ramsay G, Chawarska K, Klin A . Out of the mouths of babes: vocal production in infant siblings of children with ASD. J Child Psychol Psychiatry 2011; 52: 588–598.
Shic F, Macari S, Chawarska K . Speech disturbs face scanning in 6-month-old infants who develop autism spectrum disorder. Biol Psychiatry 2014; 75: 231–237.
Open Science Collaboration. PSYCHOLOGY. Estimating the reproducibility of psychological science. Science (New York, NY) 2015; 349: aac4716.
Shen MD, Nordahl CW, Young GS, Wootton-Gorges SL, Lee A, Liston SE et al. Early brain enlargement and elevated extra-axial fluid in infants who develop autism spectrum disorder. Brain 2013; 136: 2825–2835.
Shen MD, Styner MA, Swanson MR, Wolff JJ, Elison JT, Graves MM et al. A Longitudinal MRI Study of Subcortical Brain Development in Infants Who Develop Autism and Infants with Fragile X Syndrome. Biological psychiatry 2016 in press.
Jones W, Klin A . Attention to eyes is present but in decline in 2–6 month-olds later diagnosed with autism. Nature 2013; 504: 427–431.
Sacrey L-AR, Armstrong VL, Bryson SE, Zwaigenbaum L . Impairments to visual disengagement in autism spectrum disorder: a review of experimental studies from infancy to adulthood. Neurosci Biobehav Rev 2014; 47: 559–577.
Hayden BY, Smith DV, Platt ML . Electrophysiological correlates of default-mode processing in macaque posterior cingulate cortex. Proc Natl Acad Sci USA 2009; 106: 5948–5953.
Putnam MC, Steven MS, Doron KW, Riggall AC, Gazzaniga MS . Cortical projection topography of the human splenium: Hemispheric asymmetry and individual differences. J Cogn Neurosci 2010; 22: 1662–1669.
Deoni SC, O'Muircheartaigh J, Elison JT, Walker L, Doernberg E, Waskiewicz N et al. White matter maturation profiles through early childhood predict general cognitive ability. Brain Struct Funct 2016; 221: 1189–1203.
Swanson MR, Wolff JJ, Elison JT, Gu H, Hazlett HC, Botteron K et al. Splenium development and early spoken language in human infants. Dev Sci 2015; 20.
Gao W, Zhu H, Giovanello KS, Smith JK, Shen D, Gilmore JH et al. Evidence on the emergence of the brain's default network from 2-week-old to 2-year-old healthy pediatric subjects. Proc Natl Acad Sci USA 2009; 106: 6790–6795.
Thomas MS, Davis R, Karmiloff-Smith A, Knowland VC, Charman T . The over-pruning hypothesis of autism. Dev Sci 2016; 19: 284–305.
Cohen IL, Gardner JM, Karmel BZ, Phan HT, Kittler P, Gomez TR et al. Neonatal brainstem function and 4-month arousal-modulated attention are jointly associated with autism. Autism Res 2013; 6: 11–22.
Pryweller JR, Schauder KB, Anderson AW, Heacock JL, Foss-Feig JH, Newsom CR et al. White matter correlates of sensory processing in autism spectrum disorders. NeuroImage Clin 2014; 6: 379–387.
Nebel MB, Joel SE, Muschelli J, Barber AD, Caffo BS, Pekar JJ et al. Disruption of functional organization within the primary motor cortex in children with autism. Hum Brain Mapping 2014; 35: 567–580.
Mosconi MW, Sweeney JA . Sensorimotor dysfunctions as primary features of autism spectrum disorders. Sci China Life Sci 2015; 58: 1016–1023.
Neely KA, Mohanty S, Schmitt LM, Wang Z, Sweeney JA, Mosconi MW . Motor memory deficits contribute to motor impairments in autism spectrum disorder. J Autism Dev Disord 2016.
Floris DL, Barber AD, Nebel MB, Martinelli M, Lai MC, Crocetti D et al. Atypical lateralization of motor circuit functional connectivity in children with autism is associated with motor deficits. Mol Autism 2016; 7: 35.
Nebel MB, Eloyan A, Nettles CA, Sweeney KL, Ament K, Ward RE et al. Intrinsic visual-motor synchrony correlates with social deficits in autism. Biol Psychiatry 2016; 79: 633–641.
Green SA, Hernandez L, Bookheimer SY, Dapretto M . Salience network connectivity in autism is related to brain and behavioral markers of sensory overresponsivity. J Am Acad Child Adolesc Psychiatry. 2016; 55 7: 618–626, e1.
Green SA, Hernandez L, Tottenham N, Krasileva K, Bookheimer SY, Dapretto M . Neurobiology of Sensory Overresponsivity in Youth With Autism Spectrum Disorders. JAMA Psychiatry 2015; 72: 778–786.
Whyatt C, Craig C . Sensory-motor problems in Autism. Front Integr Neurosci 2013; 7.
Mosconi MW, Kay M, D'Cruz AM, Guter S, Kapur K, Macmillan C et al. Neurobehavioral abnormalities in first-degree relatives of individuals with autism. Arch Gen Psychiatry 2010; 67: 830–840.
Ibanez LV, Grantz CJ, Messinger DS . The development of referential communication and autism symptomatology in high-risk infants. Infancy 2013; 18: 5.
Miller M, Iosif AM, Hill M, Young GS, Schwichtenberg AJ, Ozonoff S . Response to name in infants developing autism spectrum disorder: A Prospective Study. J Pediatr 2017; 183: 141–146.
Cavanna AE, Trimble MR . The precuneus: a review of its functional anatomy and behavioural correlatesBrain 2006; 129: 564–583.
Margulies DS, Vincent JL, Kelly C, Lohmann G, Uddin LQ, Biswal BB et al. Precuneus shares intrinsic functional architecture in humans and monkeys. Proc Natl Acad Sci USA 2009; 106: 20069–20074.
Duhamel JR, Colby CL, Goldberg ME . The updating of the representation of visual space in parietal cortex by intended eye movements. Science (New York, NY) 1992; 255: 90–92.
Seltzer B, Pandya DN . Converging visual and somatic sensory cortical input to the intraparietal sulcus of the rhesus monkey. Brain Res 1980; 192: 339–351.
Wolff JJ, Gerig G, Lewis JD, Soda T, Styner MA, Vachet C et al. Altered corpus callosum morphology associated with autism over the first 2 years of lifeBrain 2015; 138: 2046–2058.
Wolff JJ SM, Elison JT, Gerig G, Pruett JR Jr., Styner MA, Vachet C et alThe IBIS Network. Neural circuitry at age 6 months associated with later repetitive behavior and sensory features in autism. Mol Autism 2017; 8: 8.
Wang Samuel SH, Kloth Alexander D, Badura A . The cerebellum, sensitive periods, and autism. Neuron 2014; 83: 518–532.
Courchesne E, Karns CM, Davis HR, Ziccardi R, Carper RA, Tigue ZD et al. Unusual brain growth patterns in early life in patients with autistic disorder: an MRI study. Neurology 2001; 57: 245–254.
Sparks BF, Friedman SD, Shaw DW, Aylward EH, Echelard D, Artru AA et al. Brain structural abnormalities in young children with autism spectrum disorder. Neurology 2002; 59: 184–192.
Hazlett HC, Poe M, Gerig G, Smith RG, Provenzale J, Ross A et al. Magnetic resonance imaging and head circumference study of brain size in autism: birth through age 2 years. Arch Gen Psychiatry 2005; 62: 1366–1376.
Hazlett HC, Poe MD, Gerig G, Styner M, Chappell C, Smith RG et al. Early brain overgrowth in autism associated with an increase in cortical surface area before age 2 years. Arch Gen Psychiatry 2011; 68: 467–476.
Ohta H, Nordahl CW, Iosif AM, Lee A, Rogers S, Amaral DG . Increased surface area, but not cortical thickness, in a subset of young boys with autism spectrum disorder. Autism Res 2016; 9: 232–248.
Hazlett H, Gu H, Munsell B, Kim S, Styner M, Wolff J et al. Early brain development in infants at high risk of autism spectrum disorder. .Nature 2017; 542: 348–351.
Lord C, Risi S, Lambrecht L, Cook EH Jr., Leventhal BL, DiLavore PC et al. The autism diagnostic observation schedule-generic: a standard measure of social and communication deficits associated with the spectrum of autism. J Autism Dev Disord 2000; 30: 205–223.
Wetherby AM, Allen L, Cleary J, Kublin K, Goldstein H . Validity and reliability of the communication and symbolic behavior scales developmental profile with very young children. J Speech Lang Hear Res 2002; 45: 1202–1218.
Betancur C . Etiological heterogeneity in autism spectrum disorders: more than 100 genetic and genomic disorders and still counting. Brain Res 2011; 1380: 42–77.
Nordahl CW, Lange N, Li DD, Barnett LA, Lee A, Buonocore MH et al. Brain enlargement is associated with regression in preschool-age boys with autism spectrum disorders. Proc Natl Acad Sci USA 2011; 108: 20195–20200.
Libero LE, Nordahl CW, Li DD, Ferrer E, Rogers SJ, Amaral DG . Persistence of megalencephaly in a subgroup of young boys with autism spectrum disorder. Autism Res 2016; 9: 1169–1182.
Amaral DG, Li D, Libero L, Solomon M, Van de Water J, Mastergeorge A et al. In pursuit of neurophenotypes: The consequences of having autism and a big brain. Autism Res 2017; 10: 711–722.
Jones KL, Jones MC . Smith's Recognizable Patterns of Human Malformation7th ednSaunders, 2013; 1016, p.
Fang WQ, Chen WW, Jiang L, Liu K, Yung WH, Fu AK et al. Overproduction of upper-layer neurons in the neocortex leads to autism-like features in mice. Cell Rep 2014; 9: 1635–1643.
Li G, Nie J, Wang L, Shi F, Lin W, Gilmore JH et al. Mapping region-specific longitudinal cortical surface expansion from birth to 2 years of age. Cereb Cortex 2013; 23: 2724–2733.
Rakic P . Radial versus tangential migration of neuronal clones in the developing cerebral cortex. Proc Natl Acad Sci USA 1995; 92: 11323–11327.
Reillo I, de Juan Romero C, Garcia-Cabezas MA, Borrell V . A role for intermediate radial glia in the tangential expansion of the mammalian cerebral cortex. Cereb Cortex 2011; 21: 1674–1694.
Lui JH, Hansen DV, Kriegstein AR . Development and evolution of the human neocortex. Cell 2011; 146: 18–36.
LaMonica BE, Lui JH, Wang X, Kriegstein AR . OSVZ progenitors in the human cortex: an updated perspective on neurodevelopmental disease. Curr Opin Neurobiol 2012; 22: 747–753.
Hansen DV, Lui JH, Parker PRL, Kriegstein AR . Neurogenic radial glia in the outer subventricular zone of human neocortex. Nature 2010; 464: 554–561.
Kriegstein A, Noctor S, Martinez-Cerdeno V . Patterns of neural stem and progenitor cell division may underlie evolutionary cortical expansion. Nat Rev Neurosci 2006; 7: 883–890.
Woodhead GJ, Mutch CA, Olson EC, Chenn A . Cell-autonomous beta-catenin signaling regulates cortical precursor proliferation. J Neurosci 2006; 26: 12620–12630.
Chenn A, Walsh CA . Regulation of cerebral cortical size by control of cell cycle exit in neural precursors. Science 2002; 297: 365–369.
Florio M, Albert M, Taverna E, Namba T, Brandl H, Lewitus E et al. Human-specific gene ARHGAP11B promotes basal progenitor amplification and neocortex expansion. Science 2015; 347: 1465–1470.
Rubenstein JL . Three hypotheses for developmental defects that may underlie some forms of autism spectrum disorder. Curr Opin Neurol 2010; 23: 118–123.
Docherty AR, Hagler DJ Jr., Panizzon MS, Neale MC, Eyler LT, Fennema-Notestine C et al. Does degree of gyrification underlie the phenotypic and genetic associations between cortical surface area and cognitive ability? NeuroImage 2015; 106: 154–160.
Chen CH, Peng Q, Schork AJ, Lo MT, Fan CC, Wang Y et al. Large-scale genomics unveil polygenic architecture of human cortical surface area. Nat Commun 2015; 6: 7549.
Chen CH, Fiecas M, Gutierrez ED, Panizzon MS, Eyler LT, Vuoksimaa E et al. Genetic topography of brain morphology. Proc Natl Acad Sci USA 2013; 110: 17089–17094.
Panizzon MS, Fennema-Notestine C, Eyler LT, Jernigan TL, Prom-Wormley E, Neale M et al. Distinct genetic influences on cortical surface area and cortical thickness. Cereb Cortex 2009; 19: 2728–2735.
Stein JL, de la Torre-Ubieta L, Tian Y, Parikshak NN, Hernandez IA, Marchetto MC et al. A quantitative framework to evaluate modeling of cortical development by neural stem cells. Neuron 2014; 83: 69–86.
Pan X, Chang X, Leung C, Zhou Z, Cao F, Xie W et al. PAK1 regulates cortical development via promoting neuronal migration and progenitor cell proliferation. Mol Brain 2015; 8: 36.
Belinson H, Nakatani J, Babineau BA, Birnbaum RY, Ellegood J, Bershteyn M et al. Prenatal beta-catenin/Brn2/Tbr2 transcriptional cascade regulates adult social and stereotypic behaviors. Mol Psychiatry 2016; 21: 1417–1433.
Packer A . Neocortical neurogenesis and the etiology of autism spectrum disorder. Neurosci Biobehav Rev 2016; 64: 185–195.
Lyall AE, Shi F, Geng X, Woolson S, Li G, Wang L et al. Dynamic development of regional cortical thickness and surface area in early childhood. Cereb Cortex 2015; 25: 2204–2212.
Courchesne E, Mouton PR, Calhoun ME, Semendeferi K, Ahrens-Barbeau C, Hallet MJ et al. Neuron number and size in prefrontal cortex of children with autism. JAMA 2011; 306: 2001–2010.
Kim E, Camacho J, Combs Z, Ariza J, Lechpammer M, Noctor SC et al. Preliminary findings suggest the number and volume of supragranular and infragranular pyramidal neurons are similar in the anterior superior temporal area of control subjects and subjects with autism. Neurosci Lett 2015; 589: 98–103.
Jacot-Descombes S, Uppal N, Wicinski B, Santos M, Schmeidler J, Giannakopoulos P et al. Decreased pyramidal neuron size in Brodmann areas 44 and 45 in patients with autism. Acta Neuropathol 2012; 124: 67–79.
Casanova MF, van Kooten IA, Switala AE, van Engeland H, Heinsen H, Steinbusch HW et al. Minicolumnar abnormalities in autism. Acta Neuropathol 2006; 112: 287–303.
McKavanagh R, Buckley E, Chance SA . Wider minicolumns in autism: a neural basis for altered processing? Brain 2015; 138: 2034–2045.
Huttenlocher PR . Morphometric study of human cerebral cortex development. Neuropsychologia 1990; 28: 517–527.
Tang G, Gudsnuk K, Kuo SH, Cotrina ML, Rosoklija G, Sosunov A et al. Loss of mTOR-dependent macroautophagy causes autistic-like synaptic pruning deficits. Neuron 2014; 83: 1131–1143.
Frazier TW, Embacher R, Tilot AK, Koenig K, Mester J, Eng C . Molecular and phenotypic abnormalities in individuals with germline heterozygous PTEN mutations and autism. Mol Psychiatry 2015; 20: 1132–1138.
Kwon CH, Luikart BW, Powell CM, Zhou J, Matheny SA, Zhang W et al. Pten regulates neuronal arborization and social interaction in mice. Neuron 2006; 50: 377–388.
Vogt D, Cho KK, Lee AT, Sohal VS, Rubenstein JL . The parvalbumin/somatostatin ratio is increased in Pten mutant mice and by human PTEN ASD alleles. Cell Rep 2015; 11: 944–956.
Fang WQ, Chen WW, Fu AK, Ip NY . Axin directs the amplification and differentiation of intermediate progenitors in the developing cerebral cortex. Neuron 2013; 79: 665–679.
Rubenstein JL, Merzenich MM . Model of autism: increased ratio of excitation/inhibition in key neural systems. Genes Brain Behav 2003; 2: 255–267.
Hensch TK . Critical period plasticity in local cortical circuits. Nat Rev Neurosci 2005; 6: 877–888.
Roullet FI, Lai JK, Foster JA . In utero exposure to valproic acid and autism--a current review of clinical and animal studies. Neurotoxicol Teratol 2013; 36: 47–56.
Fujimura K, Mitsuhashi T, Shibata S, Shimozato S, Takahashi T . In utero exposure to valproic acid induces neocortical dysgenesis via dysregulation of neural progenitor cell proliferation/differentiation. J Neurosci 2016; 36: 10908–10919.
Marchetto MC, Belinson H, Tian Y, Freitas BC, Fu C, Vadodaria KC et al. Altered proliferation and networks in neural cells derived from idiopathic autistic individuals. Mol Psychiatry 2016; 22: 820–835.
Bernier R, Golzio C, Xiong B, Stessman HA, Coe BP, Penn O et al. Disruptive CHD8 mutations define a subtype of autism early in development. Cell 2014; 158: 263–276.
Qureshi AY, Mueller S, Snyder AZ, Mukherjee P, Berman JI, Roberts TPL et al. Opposing brain differences in 16p11.2 deletion and duplication carriers. J Neurosci 2014; 34: 11199–11211.
Weiss LA, Shen Y, Korn JM, Arking DE, Miller DT, Fossdal R et al. Association between microdeletion and microduplication at 16p11.2 and autism. N Engl J Med 2008; 358: 667–675.
Pucilowska J, Vithayathil J, Tavares EJ, Kelly C, Karlo JC, Landreth GE . The 16p11.2 deletion mouse model of autism exhibits altered cortical progenitor proliferation and brain cytoarchitecture linked to the ERK MAPK pathway. J Neurosci 2015; 35: 3190–3200.
Wang P, Lin M, Pedrosa E, Hrabovsky A, Zhang Z, Guo W et al. CRISPR/Cas9-mediated heterozygous knockout of the autism gene CHD8 and characterization of its transcriptional networks in neurodevelopment. Mol Autism 2015; 6: 55.
Katayama Y, Nishiyama M, Shoji H, Ohkawa Y, Kawamura A, Sato T et al. CHD8 haploinsufficiency results in autistic-like phenotypes in mice. Nature 2016; 537: 675–679.
Durak O, Gao F, Kaeser-Woo YJ, Rueda R, Martorell AJ, Nott A et al. Chd8 mediates cortical neurogenesis via transcriptional regulation of cell cycle and Wnt signaling. Nat Neurosci 2016; 19: 1477–1488.
Lien WH, Fuchs E . Wnt some lose some: transcriptional governance of stem cells by Wnt/beta-catenin signaling. Genes Dev 2014; 28: 1517–1532.
Huang WC, Chen Y, Page DT . Hyperconnectivity of prefrontal cortex to amygdala projections in a mouse model of macrocephaly/autism syndrome. Nat Commun 2016; 7: 13421.
van Bon BW, Coe BP, Bernier R, Green C, Gerdts J, Witherspoon K et al. Disruptive de novo mutations of DYRK1A lead to a syndromic form of autism and ID. Mol Psychiatry 2016; 21: 126–132.
Wolff JJ, Gu H, Gerig G, Elison JT, Styner M, Gouttard S . Differences in white matter fiber tract development present from 6 to 24 months in infants with autism. Am J Psychiatry 2012; 169: 589–600.
Wolff J, Swanson MR, Elison JT, Gerig G, Pruett JR J, Styner MA et al. Neural circuitry at age 6 months associated with later repetitive behavior and sensory features in autism. Mol Autism 2016; 8: 8, in revision.
Johanson CE, Duncan JA 3rd, Klinge PM, Brinker T, Stopa EG, Silverberg GD . Multiplicity of cerebrospinal fluid functions: New challenges in health and disease. Cerebrospinal Fluid Res 2008; 5: 10.
Iliff JJ, Wang M, Liao Y, Plogg BA, Peng W, Gundersen GA et al. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid beta. Sci Transl Med 2012; 4: 147ra11.
Nickel RE, Gallenstein JS . Developmental prognosis for infants with benign enlargement of the subarachnoid spaces. Dev Med Child Neurol 1987; 29: 181–186.
Lorch SA, D'Agostino J, Zimmerman R, Bernbaum J . "benign" extra-axial fluid in survivors of neonatal intensive care. Arch Pediatr Adolesc Med 2004; 158: 178–182.
Hellbusch LC . Benign extracerebral fluid collections in infancy: clinical presentation and long-term follow-up. J Neurosurg 2007; 107: 119–125.
Lehtinen MK, Zappaterra MW, Chen X, Yang YJ, Hill AD, Lun M et al. The cerebrospinal fluid provides a proliferative niche for neural progenitor cells. Neuron 2011; 69: 893–905.
Orekhova EV, Elsabbagh M, Jones EJ, Dawson G, Charman T, Johnson MH . EEG hyper-connectivity in high-risk infants is associated with later autism. J Neurodev Disord 2014; 6: 40.
Jones EJ, Venema K, Earl R, Lowy R, Barnes K, Estes A et al. Reduced engagement with social stimuli in 6-month-old infants with later autism spectrum disorder: a longitudinal prospective study of infants at high familial risk. J Neurodev Disord 2016; 8: 7.
Johnson MH . Interactive specialization: a domain-general framework for human functional brain development? Dev Cogn Neurosci 2011; 1: 7–21.
Zielinski BA, Prigge MB, Nielsen JA, Froehlich AL, Abildskov TJ, Anderson JS et al. Longitudinal changes in cortical thickness in autism and typical development. Brain 2014; 137: 1799–1812.
Ecker C, Ginestet C, Feng Y, Johnston P, Lombardo MV, Lai MC et al. Brain surface anatomy in adults with autism: the relationship between surface area, cortical thickness, and autistic symptoms. JAMA Psychiatry 2013; 70: 59–70.
Chevallier C, Kohls G, Troiani V, Brodkin ES, Schultz RT . The social motivation theory of autism. Trends Cogn Sci 2012; 16: 231–239.
Bergmann J, Pilatus U, Genc E, Kohler A, Singer W, Pearson J . V1 surface size predicts GABA concentration in medial occipital cortex. NeuroImage 2016; 124: 654–662.
Mariani J, Coppola G, Zhang P, Abyzov A, Provini L, Tomasini L et al. FOXG1-dependent dysregulation of gaba/glutamate neuron differentiation in autism spectrum disorders. Cell 2015; 162: 375–390.
Hensch TK . Critical period mechanisms in developing visual cortex. Curr Top Dev Biol 2005; 69: 215–237.
LeBlanc JJ, Fagiolini M . Autism: a "critical period" disorder? Neural Plast 2011; 2011: 921680.
Yashiro K, Riday TT, Condon KH, Roberts AC, Bernardo DR, Prakash R et al. Ube3a is required for experience-dependent maturation of the neocortex. Nat Neurosci 2009; 12: 777–783.
Rogers SJ, Vismara L, Wagner AL, McCormick C, Young G, Ozonoff S . Autism treatment in the first year of life: a pilot study of infant start, a parent-implemented intervention for symptomatic infants. J Autism Dev Disord 2014; 44: 2981–2995.
Wass S, Porayska-Pomsta K, Johnson MH . Training attentional control in infancy. Curr Biol 2011; 21: 1543–1547.
Acknowledgements
This work was supported by an NIH Autism Center of Excellence grant (NIMH and NICHD #HD055741 to J.P); NIMH #MH104324 (JTE), the Angelman Syndrome Foundation (MJZ) and a NIH Pioneer Award DP1ES024088 (MJZ).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
PowerPoint slides
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/4.0/
About this article
Cite this article
Piven, J., Elison, J. & Zylka, M. Toward a conceptual framework for early brain and behavior development in autism. Mol Psychiatry 22, 1385–1394 (2017). https://doi.org/10.1038/mp.2017.131
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/mp.2017.131
This article is cited by
-
Examining Autism Spectrum Using the Attention Network Test: A Meta-Analysis
Review Journal of Autism and Developmental Disorders (2024)
-
White matter development and language abilities during infancy in autism spectrum disorder
Molecular Psychiatry (2024)
-
The early life growth of head circumference, weight, and height in infants with autism spectrum disorders: a systematic review
BMC Pediatrics (2023)
-
Pathways to Psychopathology Among Autistic Adults
Current Psychiatry Reports (2023)
-
Validation of the Modified Checklist for Autism in Toddlers, Revised with Follow-up in a Population Sample of 30-Month-Old Children in Iceland: A Prospective Approach
Journal of Autism and Developmental Disorders (2022)