Abstract
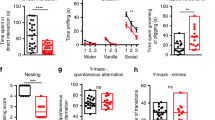
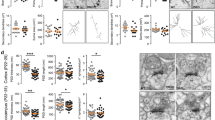
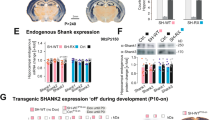
SHANK3 (also called PROSAP2) genetic haploinsufficiency is thought to be the major cause of neuropsychiatric symptoms in Phelan-McDermid syndrome (PMS). PMS is a rare genetic disorder that causes a severe form of intellectual disability (ID), expressive language delays and other autistic features. Furthermore, a significant number of SHANK3 mutations have been identified in patients with autism spectrum disorders (ASD), and SHANK3 truncating mutations are associated with moderate to profound ID. The Shank3 protein is a scaffold protein that is located in the postsynaptic density (PSD) of excitatory synapses and is crucial for synapse development and plasticity. In this study, we investigated the molecular mechanisms associated with the ASD-like behaviors observed in Shank3Δ11−/− mice, in which exon 11 has been deleted. Our results indicate that Shank3 is essential to mediating metabotropic glutamate receptor 5 (mGlu5)-receptor signaling by recruiting Homer1b/c to the PSD, specifically in the striatum and cortex. Moreover, augmenting mGlu5-receptor activity by administering 3-Cyano-N-(1,3-diphenyl-1H-pyrazol-5-yl)benzamide ameliorated the functional and behavioral defects that were observed in Shank3Δ11−/− mice, suggesting that pharmaceutical treatments that increase mGlu5 activity may represent a new approach for treating patients that are affected by PMS and SHANK3 mutations.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Phelan K, McDermid HE . The 22q13.3 Deletion syndrome (Phelan-McDermid Syndrome). Mol Syndromol 2012; 2: 186–201.
Durand CM, Betancur C, Boeckers TM, Bockmann J, Chaste P, Fauchereau F et al. Mutations in the gene encoding the synaptic scaffolding protein SHANK3 are associated with autism spectrum disorders. Nat Genet 2007; 39: 25–27.
Bozdagi O, Sakurai T, Papapetrou D, Wang X, Dickstein DL, Takahashi N et al. Haploinsufficiency of the autism-associated Shank3 gene leads to deficits in synaptic function, social interaction, and social communication. Mol Autism 2010; 1: 15.
Peça J, Feliciano C, Ting JT, Wang W, Wells MF, Venkatraman TN et al. Shank3 mutant mice display autistic-like behaviours and striatal dysfunction. Nature 2011; 472: 437–442.
Wang X, McCoy PA, Rodriguiz RM, Pan Y, Je HS, Roberts AC et al. Synaptic dysfunction and abnormal behaviors in mice lacking major isoforms of Shank3. Hum Mol Genet 2011; 20: 3093–3108.
Yang M, Bozdagi O, Scattoni ML, Wöhr M, Roullet FI, Katz AM et al. Reduced excitatory neurotransmission and mild autism-relevant phenotypes in adolescent Shank3 null mutant mice. J Neurosci 2012; 32: 6525–6541.
Kouser M, Speed HE, Dewey CM, Reimers JM, Widman AJ, Gupta N et al. Loss of predominant Shank3 isoforms results in hippocampus-dependent impairments in behavior and synaptic transmission. J Neurosci 2013; 33: 18448–18468.
Speed HE, Kouser M, Xuan Z, Reimers JM, Ochoa CF, Gupta N et al. Autism-Associated Insertion Mutation (InsG) of Shank3 Exon 21 Causes Impaired Synaptic Transmission and Behavioral Deficits. J Neurosci 2015; 35: 9648–9665.
Jiang YH, Ehlers MD . Modeling autism by SHANK gene mutations in mice. Neuron 2013; 78: 8–27.
Sala C, Roussignol G, Meldolesi J, Fagni L . Key role of the postsynaptic density scaffold proteins shank and homer in the functional architecture of Ca2+ homeostasis at dendritic spines in hippocampal neurons. J Neurosci 2005; 25: 4587–4592.
Sala C, Piech V, Wilson NR, Passafaro M, Liu GS, Sheng M . Regulation of dendritic spine morphology and synaptic function by Shank and Homer. Neuron 2001; 31: 115–130.
Hung AY, Futai K, Sala C, Valtschanoff JG, Ryu J, Woodworth MA et al. Smaller dendritic spines, weaker synaptic transmission, but enhanced spatial learning in mice lacking Shank1. J Neurosci 2008; 28: 1697–1708.
Roussignol G, Ango F, Romorini S, Tu JC, Sala C, Worley PF et al. Shank expression is sufficient to induce functional dendritic spine synapses in aspiny neurons. J Neurosci 2005; 25: 3560–3570.
Verpelli C, Dvoretskova E, Vicidomini C, Rossi F, Chiappalone M, Schoen M et al. Importance of Shank3 protein in regulating metabotropic glutamate receptor 5 (mGluR5) expression and signaling at synapses. J Biol Chem 2011; 286: 34839–34850.
Naisbitt S, Kim E, Tu JC, Xiao B, Sala C, Valtschanoff J et al. Shank, a novel family of postsynaptic density proteins that binds to the NMDA receptor/PSD-95/GKAP complex and cortactin. Neuron 1999; 23: 569–582.
Tu JC, Xiao B, Naisbitt S, Yuan JP, Petralia RS, Brakeman P et al. Coupling of mGluR/Homer and PSD-95 complexes by the Shank family of postsynaptic density proteins. Neuron 1999; 23: 583–592.
Boeckers TM, Kreutz MR, Winter C, Zuschratter W, Smalla KH, Sanmarti-Vila L et al. Proline-rich synapse-associated protein-1/cortactin binding protein 1 (ProSAP1/CortBP1) is a PDZ-domain protein highly enriched in the postsynaptic density. J Neurosci 1999; 19: 6506–6518.
Boeckers TM, Winter C, Smalla KH, Kreutz MR, Bockmann J, Seidenbecher C et al. Proline-rich synapse-associated proteins ProSAP1 and ProSAP2 interact with synaptic proteins of the SAPAP/GKAP family. Biochem Biophys Res Commun 1999; 264: 247–252.
Chana G, Laskaris L, Pantelis C, Gillett P, Testa R, Zantomio D et al. Decreased expression of mGluR5 within the dorsolateral prefrontal cortex in autism and increased microglial number in mGluR5 knockout mice: Pathophysiological and neurobehavioral implications. Brain Behav Immun 2015; 49: 197–205.
Giuffrida R, Musumeci S, D'Antoni S, Bonaccorso CM, Giuffrida-Stella AM, Oostra BA et al. A reduced number of metabotropic glutamate subtype 5 receptors are associated with constitutive homer proteins in a mouse model of fragile X syndrome. J Neurosci 2005; 25: 8908–8916.
Ronesi JA, Huber KM . Homer interactions are necessary for metabotropic glutamate receptor-induced long-term depression and translational activation. J Neurosci 2008; 28: 543–547.
Lujan R, Roberts JD, Shigemoto R, Ohishi H, Somogyi P . Differential plasma membrane distribution of metabotropic glutamate receptors mGluR1 alpha, mGluR2 and mGluR5, relative to neurotransmitter release sites. J Chem Neuroanat 1997; 13: 219–241.
Shigemoto R, Nomura S, Ohishi H, Sugihara H, Nakanishi S, Mizuno N . Immunohistochemical localization of a metabotropic glutamate receptor, mGluR5, in the rat brain. Neurosci Lett 1993; 163: 53–57.
Romano C, Sesma MA, McDonald CT, O'Malley K, Van den Pol AN, Olney JW . Distribution of metabotropic glutamate receptor mGluR5 immunoreactivity in rat brain. J Comp Neurol 1995; 355: 455–469.
Schmeisser MJ, Ey E, Wegener S, Bockmann J, Stempel AV, Kuebler A et al. Autistic-like behaviours and hyperactivity in mice lacking ProSAP1/Shank2. Nature 2012; 486: 256–260.
Giustetto M, Kirsch J, Fritschy JM, Cantino D, Sassoè-Pognetto M . Localization of the clustering protein gephyrin at GABAergic synapses in the main olfactory bulb of the rat. J Comp Neurol 1998; 395: 231–244.
Distler U, Schmeisser MJ, Pelosi A, Reim D, Kuharev J, Weiczner R et al. In-depth protein profiling of the postsynaptic density from mouse hippocampus using data-independent acquisition proteomics. Proteomics 2014; 14: 2607–2613.
Calabresi P, Pisani A, Mercuri NB, Bernardi G . Long-term Potentiation in the Striatum is Unmasked by Removing the Voltage-dependent Magnesium Block of NMDA Receptor Channels. Eur J Neurosci 1992; 4: 929–935.
Sala M, Braida D, Lentini D, Busnelli M, Bulgheroni E, Capurro V et al. Pharmacologic rescue of impaired cognitive flexibility, social deficits, increased aggression, and seizure susceptibility in oxytocin receptor null mice: a neurobehavioral model of autism. Biol Psychiatry 2011; 69: 875–882.
McFarlane HG, Kusek GK, Yang M, Phoenix JL, Bolivar VJ, Crawley JN . Autism-like behavioral phenotypes in BTBR T+tf/J mice. Genes Brain Behav 2008; 7: 152–163.
Kenney JW, Adoff MD, Wilkinson DS, Gould TJ . The effects of acute, chronic, and withdrawal from chronic nicotine on novel and spatial object recognition in male C57BL/6 J mice. Psychopharmacology (Berl) 2011; 217: 353–365.
Bevins RA, Besheer J . Object recognition in rats and mice: a one-trial non-matching-to-sample learning task to study 'recognition memory'. Nat Protoc 2006; 1: 1306–1311.
Morris R . Developments of a water-maze procedure for studying spatial learning in the rat. J Neurosci Methods 1984; 11: 47–60.
Zhang H, Zhang SB, Zhang QQ, Liu M, He XY, Zou Z et al. Rescue of cAMP response element-binding protein signaling reversed spatial memory retention impairments induced by subanesthetic dose of propofol. CNS Neurosci Ther 2013; 19: 484–493.
Verpelli C, Carlessi L, Bechi G, Fusar Poli E, Orellana D, Heise C et al. Comparative neuronal differentiation of self-renewing neural progenitor cell lines obtained from human induced pluripotent stem cells. Front Cell Neurosci 2013; 7: 175.
Charan J, Kantharia ND . How to calculate sample size in animal studies? J Pharmacol Pharmacother 2013; 4: 303–306.
Kammermeier PJ, Xiao B, Tu JC, Worley PF, Ikeda SR . Homer proteins regulate coupling of group I metabotropic glutamate receptors to N-type calcium and M-type potassium channels. J Neurosci 2000; 20: 7238–7245.
Kammermeier PJ, Worley PF . Homer 1a uncouples metabotropic glutamate receptor 5 from postsynaptic effectors. Proc Natl Acad Sci USA 2007; 104: 6055–6060.
D'Antoni S, Spatuzza M, Bonaccorso CM, Musumeci SA, Ciranna L, Nicoletti F et al. Dysregulation of group-I metabotropic glutamate (mGlu) receptor mediated signalling in disorders associated with Intellectual Disability and Autism. Neurosci Biobehav Rev 2014; 46 (Pt 2): 228–241.
Hayashi MK, Tang C, Verpelli C, Narayanan R, Stearns MH, Xu RM et al. The postsynaptic density proteins Homer and Shank form a polymeric network structure. Cell 2009; 137: 159–171.
Verpelli C, Sala C . Molecular and synaptic defects in intellectual disability syndromes. Curr Opin Neurobiol 2012; 22: 530–536.
Zitzer H, Honck HH, Bachner D, Richter D, Kreienkamp HJ . Somatostatin receptor interacting protein defines a novel family of multidomain proteins present in human and rodent brain. J Biol Chem 1999; 274: 32997–33001.
Zitzer H, Richter D, Kreienkamp HJ . Agonist-dependent interaction of the rat somatostatin receptor subtype 2 with cortactin-binding protein 1. J Biol Chem 1999; 274: 18153–18156.
Kinney GG, O'Brien JA, Lemaire W, Burno M, Bickel DJ, Clements MK et al. A novel selective positive allosteric modulator of metabotropic glutamate receptor subtype 5 has in vivo activity and antipsychotic-like effects in rat behavioral models. J Pharmacol Exp Ther 2005; 313: 199–206.
Pisani A, Gubellini P, Bonsi P, Conquet F, Picconi B, Centonze D et al. Metabotropic glutamate receptor 5 mediates the potentiation of N-methyl-D-aspartate responses in medium spiny striatal neurons. Neuroscience 2001; 106: 579–587.
Bonsi P, Platania P, Martella G, Madeo G, Vita D, Tassone A et al. Distinct roles of group I mGlu receptors in striatal function. Neuropharmacology 2008; 55: 392–395.
Lindsley CW, Wisnoski DD, Leister WH, O'brien JA, Lemaire W, Williams DL et al. Discovery of positive allosteric modulators for the metabotropic glutamate receptor subtype 5 from a series of N-(1,3-diphenyl-1H- pyrazol-5-yl)benzamides that potentiate receptor function in vivo. J Med Chem 2004; 47: 5825–5828.
Uslaner JM, Parmentier-Batteur S, Flick RB, Surles NO, Lam JS, McNaughton CH et al. Dose-dependent effect of CDPPB, the mGluR5 positive allosteric modulator, on recognition memory is associated with GluR1 and CREB phosphorylation in the prefrontal cortex and hippocampus. Neuropharmacology 2009; 57: 531–538.
Bozdagi O, Tavassoli T, Buxbaum JD . Insulin-like growth factor-1 rescues synaptic and motor deficits in a mouse model of autism and developmental delay. Mol Autism 2013; 4: 9.
Drapeau E, Dorr NP, Elder GA, Buxbaum JD . Absence of strong strain effects in behavioral analyses of Shank3-deficient mice. Dis Model Mech 2014; 7: 667–681.
Lee J, Chung C, Ha S, Lee D, Kim DY, Kim H et al. Shank3-mutant mice lacking exon 9 show altered excitation/inhibition balance, enhanced rearing, and spatial memory deficit. Front Cell Neurosci 2015; 9: 94.
Welch JM, Lu J, Rodriguiz RM, Trotta NC, Peca J, Ding JD et al. Cortico-striatal synaptic defects and OCD-like behaviours in Sapap3-mutant mice. Nature 2007; 448: 894–900.
Langen M, Bos D, Noordermeer SD, Nederveen H, van Engeland H, Durston S . Changes in the development of striatum are involved in repetitive behavior in autism. Biol Psychiatry 2014; 76: 405–411.
Duffney LJ, Zhong P, Wei J, Matas E, Cheng J, Qin L et al. Autism-like Deficits in Shank3-Deficient Mice Are Rescued by Targeting Actin Regulators. Cell Rep 2015; 11: 1400–1413.
Ronesi JA, Collins KA, Hays SA, Tsai NP, Guo W, Birnbaum SG et al. Disrupted Homer scaffolds mediate abnormal mGluR5 function in a mouse model of fragile X syndrome. Nat Neurosci 2012; 15: 431–440.
Pignatelli M, Piccinin S, Molinaro G, Di Menna L, Riozzi B, Cannella M et al. Changes in mGlu5 receptor-dependent synaptic plasticity and coupling to homer proteins in the hippocampus of Ube3A hemizygous mice modeling angelman syndrome. J Neurosci 2014; 34: 4558–4566.
Kelleher RJ, Geigenmüller U, Hovhannisyan H, Trautman E, Pinard R, Rathmell B et al. High-throughput sequencing of mGluR signaling pathway genes reveals enrichment of rare variants in autism. PLoS One 2012; 7: e35003.
Won H, Lee HR, Gee HY, Mah W, Kim JI, Lee J et al. Autistic-like social behaviour in Shank2-mutant mice improved by restoring NMDA receptor function. Nature 2012; 486: 261–265.
Auerbach BD, Osterweil EK, Bear MF . Mutations causing syndromic autism define an axis of synaptic pathophysiology. Nature 2011; 480: 63–68.
Lin CW, Chen CY, Cheng SJ, Hu HT, Hsueh YP . Sarm1 deficiency impairs synaptic function and leads to behavioral deficits, which can be ameliorated by an mGluR allosteric modulator. Front Cell Neurosci 2014; 8: 87.
Conn PJ, Lindsley CW, Meiler J, Niswender CM . Opportunities and challenges in the discovery of allosteric modulators of GPCRs for treating CNS disorders. Nat Rev Drug Discov 2014; 13: 692–708.
Acknowledgements
This work was supported by the Comitato Telethon Fondazione Onlus (grant no. GGP13187 to CS and GGP11095 to CS and MG), Italian Institute of Technology (seed grant to CS), Foundation Jérôme Lejeune (to CV) and the Fondazione CARIPLO (project number 2012-0593 to CS and 2013-0879 to CV). TMB received support from the Innovative Medicines Initiative Joint Undertaking under grant agreement number 115300, the resources of which are composed of a financial contribution from the European Union’s Seventh Framework Programme (FP7/2007-2013) and a generous contribution from the EFPIA companies. MJS was supported by Baustein 3.2 of Ulm University (L.SBN.0081) and by the DAAD (PROCOPE 57049403).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Molecular Psychiatry website
Supplementary information
Rights and permissions
About this article
Cite this article
Vicidomini, C., Ponzoni, L., Lim, D. et al. Pharmacological enhancement of mGlu5 receptors rescues behavioral deficits in SHANK3 knock-out mice. Mol Psychiatry 22, 689–702 (2017). https://doi.org/10.1038/mp.2016.30
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/mp.2016.30
This article is cited by
-
Immune activation during pregnancy exacerbates ASD-related alterations in Shank3-deficient mice
Molecular Autism (2023)
-
Shank3 deletion in PV neurons is associated with abnormal behaviors and neuronal functions that are rescued by increasing GABAergic signaling
Molecular Autism (2023)
-
mGluR5 PAMs rescue cortical and behavioural defects in a mouse model of CDKL5 deficiency disorder
Neuropsychopharmacology (2023)
-
Group I and group II metabotropic glutamate receptors are upregulated in the synapses of infant rats prenatally exposed to valproic acid
Psychopharmacology (2023)
-
Signalling pathways in autism spectrum disorder: mechanisms and therapeutic implications
Signal Transduction and Targeted Therapy (2022)