Abstract
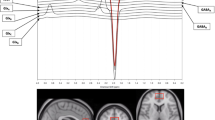
The N-methyl-D-aspartate receptor antagonist ketamine can improve major depressive disorder (MDD) within hours. To evaluate the putative role of glutamatergic and GABAergic systems in ketamine’s antidepressant action, medial prefrontal cortical (mPFC) levels of glutamate+glutamine (Glx) and γ-aminobutyric acid (GABA) were measured before, during, and after ketamine administration using proton magnetic resonance spectroscopy. Ketamine (0.5 mg kg−1 intravenously) was administered to 11 depressed patients with MDD. Glx and GABA mPFC responses were measured as ratios relative to unsuppressed voxel tissue water (W) successfully in 8/11 patients. Ten of 11 patients remitted (50% reduction in 24-item Hamilton Depression Rating Scale and total score ⩽10) within 230 min of commencing ketamine. mPFC Glx/W and GABA/W peaked at 37.8%±7.5% and 38.0%±9.1% above baseline in ~26 min. Mean areas under the curve for Glx/W (P=0.025) and GABA/W (P=0.005) increased and correlated (r=0.796; P=0.018). Clinical improvement correlated with 90-min norketamine concentration (df=6, r=−0.78, P=0.023), but no other measures.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Li N, Lee B, Liu RJ, Banasr M, Dwyer JM, Iwata M et al. mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science 2010; 329: 959–964.
Homayoun H, Moghaddam B . NMDA receptor hypofunction produces opposite effects on prefrontal cortex interneurons and pyramidal neurons. J Neuroimmune Pharmacol 2007; 27: 11496–11500.
McGirr A, Berlim MT, Bond DJ, Fleck MP, Yatham LN, Lam RW . A systematic review and meta-analysis of randomized, double-blind, placebo-controlled trials of ketamine in the rapid treatment of major depressive episodes. Psychol Med 2014; 1–12.
Fond G, Loundou A, Rabu C, Macgregor A, Lancon C, Brittner M et al. Ketamine administration in depressive disorders: a systematic review and meta-analysis. Psychopharmacology 2014; 231: 3663–3676.
Zarate CA Jr., Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA et al. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry 2006; 63: 856–864.
Murrough JW, Perez AM, Pillemer S, Stern J, Parides MK, aan het Rot M et al. Rapid and longer-term antidepressant effects of repeated ketamine infusions in treatment-resistant major depression. Biol Psychiatry 2013; 74: 250–256.
Shiroma PR, Johns B, Kuskowski M, Wels J, Thuras P, Albott CS et al. Augmentation of response and remission to serial intravenous subanesthetic ketamine in treatment resistant depression. J Affect Disord 2014; 155: 123–129.
Maeng S, Zarate CA Jr., Du J, Schloesser RJ, McCammon J, Chen G et al. Cellular mechanisms underlying the antidepressant effects of ketamine: role of alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptors. Biol Psychiatry 2008; 63: 349–352.
Koike H, Iijima M, Chaki S . Involvement of AMPA receptor in both the rapid and sustained antidepressant-like effects of ketamine in animal models of depression. Behav Brain Res 2011; 224: 107–111.
Tang SJ, Reis G, Kang H, Gingras AC, Sonenberg N, Schuman EM . A rapamycin-sensitive signaling pathway contributes to long-term synaptic plasticity in the hippocampus. Proc Natl Acad Sci USA 2002; 99: 467–472.
Lenz G, Avruch J . Glutamatergic regulation of the p70S6 kinase in primary mouse neurons. J Biol Chem 2005; 280: 38121–38124.
Cammalleri M, Lutjens R, Berton F, King AR, Simpson C, Francesconi W et al. Time-restricted role for dendritic activation of the mTOR-p70S6K pathway in the induction of late-phase long-term potentiation in the CA1. Proc Natl Acad Sci USA 2003; 100: 14368–14373.
Cracco JB, Serrano P, Moskowitz SI, Bergold PJ, Sacktor TC . Protein synthesis-dependent LTP in isolated dendrites of CA1 pyramidal cells. Hippocampus 2005; 15: 551–556.
Hou L, Klann E . Activation of the phosphoinositide 3-kinase-Akt-mammalian target of rapamycin signaling pathway is required for metabotropic glutamate receptor-dependent long-term depression. J Neurosci 2004; 24: 6352–6361.
Tischmeyer W, Schicknick H, Kraus M, Seidenbecher CI, Staak S, Scheich H et al. Rapamycin-sensitive signalling in long-term consolidation of auditory cortex-dependent memory. Eur J Neurosci 2003; 18: 942–950.
Jernigan CS, Goswami DB, Austin MC, Iyo AH, Chandran A, Stockmeier CA et al. The mTOR signaling pathway in the prefrontal cortex is compromised in major depressive disorder. Prog Neuropsychopharmacol Biol Psychiatry 2011; 35: 1774–9.
Rowland LM, Bustillo JR, Mullins PG, Jung RE, Lenroot R, Landgraf E et al. Effects of ketamine on anterior cingulate glutamate metabolism in healthy humans: a 4-T proton MRS study. Am J Psychiatry 2005; 162: 394–396.
Taylor MJ, Tiangga ER, Mhuircheartaigh RN, Cowen PJ . Lack of effect of ketamine on cortical glutamate and glutamine in healthy volunteers: a proton magnetic resonance spectroscopy study. J Psychopharmacol 2012; 26: 733–737.
Stone JM, Dietrich C, Edden R, Mehta MA, De Simoni S, Reed LJ et al. Ketamine effects on brain GABA and glutamate levels with 1H-MRS: relationship to ketamine-induced psychopathology. Mol Psychiatry 2012; 17: 664–665.
Valentine GW, Mason GF, Gomez R, Fasula M, Watzl J, Pittman B et al. The antidepressant effect of ketamine is not associated with changes in occipital amino acid neurotransmitter content as measured by [(1)H]-MRS. Psychiatry Res 2011; 191: 122–127.
Moghaddam B, Adams B, Verma A, Daly D . Activation of glutamatergic neurotransmission by ketamine: a novel step in the pathway from NMDA receptor blockade to dopaminergic and cognitive disruptions associated with the prefrontal cortex. J Neurosci 1997; 17: 2921–2927.
Chowdhury GM, Behar KL, Cho W, Thomas MA, Rothman DL, Sanacora G . (1)H-[(1)(3)C]-nuclear magnetic resonance spectroscopy measures of ketamine's effect on amino acid neurotransmitter metabolism. Biol Psychiatry 2012; 71: 1022–1025.
Price RB, Shungu DC, Mao X, Nestadt P, Kelly C, Collins KA et al. Amino acid neurotransmitters assessed by proton magnetic resonance spectroscopy: relationship to treatment resistance in major depressive disorder. Biol Psychiatry 2009; 65: 792–800.
Hasler G, van der Veen JW, Tumonis T, Meyers N, Shen J, Drevets WC . Reduced prefrontal glutamate/glutamine and gamma-aminobutyric acid levels in major depression determined using proton magnetic resonance spectroscopy. Arch Gen Psychiatry 2007; 64: 193–200.
Sanacora G, Gueorguieva R, Epperson CN, Wu YT, Appel M, Rothman DL et al. Subtype-specific alterations of gamma-aminobutyric acid and glutamate in patients with major depression. Arch Gen Psychiatry 2004; 61: 705–713.
Gold BI, Bowers MB Jr., Roth RH, Sweeney DW . GABA levels in CSF of patients with psychiatric disorders. Am J Psychiatry 1980; 137: 362–364.
Sanacora G . Cortical inhibition, gamma-aminobutyric acid, and major depression: there is plenty of smoke but is there fire? Biol Psychiatry 2010; 67: 397–398.
Sanacora G, Saricicek A . GABAergic contributions to the pathophysiology of depression and the mechanism of antidepressant action. CNS Neurol Disord Drug Targets 2007; 6: 127–140.
Sanacora G, Gueorguieva R, Epperson CN, Wu YT, Appel M, Rothman DL et al. Subtype-specific alterations of gamma-aminobutyric acid and glutamate in patients with major depression. Arch Gen Psychiatry 2004; 61: 705–713.
Sanacora G, Mason GF, Rothman DL, Behar KL, Hyder F, Petroff OA et al. Reduced cortical gamma-aminobutyric acid levels in depressed patients determined by proton magnetic resonance spectroscopy. Arch Gen Psychiatry 1999; 56: 1043–1047.
Levinson AJ, Fitzgerald PB, Favalli G, Blumberger DM, Daigle M, Daskalakis ZJ . Evidence of cortical inhibitory deficits in major depressive disorder. Biol Psychiatry 2010; 67: 458–464.
McNair DM, Lorr M, Droppleman LF . Manual for the Profile of Mood States. Education and Testing Service: San Diego, CA, USA, 1971.
Rothman DL, Petroff OA, Behar KL, Mattson RH . Localized 1H NMR measurements of gamma-aminobutyric acid in human brain in vivo. Proc Natl Acad Sci USA 1993; 90: 5662–5666.
Sailasuta N, LeRoux P, Hurd R, Wang P, Sachs N, Ketter T . Detection of cerebral gamma-aminobutyric acid (GABA) in bipolar disorder patients and healthy volunteers at 3 T. Proc Intl Soc Magn Reson Med 2001; 9: 1011.
Mori K, Hashimoto T, Harada M, Yoneda Y, Shimakawa S, Fujii E et al. [Proton magnetic resonance spectroscopy of the autistic brain]. No To Hattatsu 2001; 33: 329–335.
Dong Z . Proton MRS and MRSI of the brain without water suppression. Prog Nucl Magn Reson Spectrosc 2015; 86-87: 65–79.
Dorrius MD, Pijnappel RM, van der Weide Jansen MC, Jansen L, Kappert P, Oudkerk M et al. The added value of quantitative multi-voxel MR spectroscopy in breast magnetic resonance imaging. Eur Radiol 2012; 22: 915–922.
De Silva SS, Payne GS, Morgan VA, Ind TE, Shepherd JH, Barton DP et al. Epithelial and stromal metabolite changes in the transition from cervical intraepithelial neoplasia to cervical cancer: an in vivo 1H magnetic resonance spectroscopic imaging study with ex vivo correlation. Eur Radiol 2009; 19: 2041–2048.
Baker EH, Basso G, Barker PB, Smith MA, Bonekamp D, Horska A . Regional apparent metabolite concentrations in young adult brain measured by (1)H MR spectroscopy at 3 Tesla. J Magn Reson Imaging 2008; 27: 489–499.
Labak M, Foniok T, Kirk D, Rushforth D, Tomanek B, Jasinski A et al. Metabolic changes in rat brain following intracerebroventricular injections of streptozotocin: a model of sporadic Alzheimer's disease. Acta Neurochir Suppl 2010; 106: 177–181.
Lin JM, Chuang TC, Chung HW, Tsai SY . Quantitative comparison of post-processing methods for reduction of frequency modulation sidebands in non-water suppression 1H MRS. NMR Biomed 2013; 26: 400–409.
Lin JM, Tsai SY, Liu HS, Chung HW, Mulkern RV, Cheng CM et al. Quantification of non-water-suppressed MR spectra with correction for motion-induced signal reduction. Magn Reson Med 2009; 62: 1394–1403.
Nery FG, Stanley JA, Chen HH, Hatch JP, Nicoletti MA, Monkul ES et al. Normal metabolite levels in the left dorsolateral prefrontal cortex of unmedicated major depressive disorder patients: a single voxel (1)H spectroscopy study. Psychiatry Res 2009; 174: 177–183.
Pohl C, Block W, Karitzky J, Traber F, Schmidt S, Grothe C et al. Proton magnetic resonance spectroscopy of the motor cortex in 70 patients with amyotrophic lateral sclerosis. Arch Neurol 2001; 58: 729–735.
Zhang Y, Marenco S, Shen J . Correction of frequency and phase variations induced by eddy currents in localized spectroscopy with multiple echo times. Magn Reson Med 2007; 58: 174–178.
Kegeles LS, Mao X, Dyke J, Gonzales R, Soones T, Shungu DC . Test-retest reliability of dorsolateral prefrontal cortical GABA measurement using an 8-channel phased-array head coil with the J-editing technique at 3T. Proc Intl Soc Mag Reson Med 2006; 14: 489.
Guy W . National Institute of Mental Health (U.S.). Psychopharmacology Research Branch., Early Clinical Drug Evaluation Program. ECDEU assessment manual for psychopharmacology, Rev. edn. U. S. Dept. of Health, Education, and Welfare, Public Health Service, Alcohol, Drug Abuse, and Mental Health Administration, National Institute of Mental Health, Psychopharmacology Research Branch, Division of Extramural Research Programs: Rockville, Md. 1976; 603.
Lapidus KAB, Mathew SJ Ketamine in treatment-resistant depression In: Mann JJ, Roose SP, McGrath PJ (eds) Clinical handbook for the management of mood disorders 1st edition. edn. Cambridge University Press: Cambridge: Cambridge, 2013 pp 345–357.
Nugent AC, Diazgranados N, Carlson PJ, Ibrahim L, Luckenbaugh DA, Brutsche N et al. Neural correlates of rapid antidepressant response to ketamine in bipolar disorder. Bipolar Disord 2014; 16: 119–128.
Brady ST, Siegel GJ, Albers RW, Price DL, Benjamins J . Basic neurochemistry: principles of molecular, cellular, and medical neurobiology 8th edn. Elsevier/Academic Press: Amsterdam; Boston: Amsterdam; Boston, 2012 xxiv 1096, pp.
Bernard J, Ohayon M, Massicotte G . Modulation of the AMPA receptor by phospholipase A2: effect of the antidepressant trimipramine. Psychiatry Res 1994; 51: 107–114.
Lauterborn JC, Lynch G, Vanderklish P, Arai A, Gall CM . Positive modulation of AMPA receptors increases neurotrophin expression by hippocampal and cortical neurons. J Neurosci 2000; 20: 8–21.
Legutko B, Li X, Skolnick P . Regulation of BDNF expression in primary neuron culture by LY392098, a novel AMPA receptor potentiator. Neuropharmacology 2001; 40: 1019–1027.
Mackowiak M, O'Neill MJ, Hicks CA, Bleakman D, Skolnick P . An AMPA receptor potentiator modulates hippocampal expression of BDNF: an in vivo study. Neuropharmacology 2002; 43: 1–10.
Li X, Tizzano JP, Griffey K, Clay M, Lindstrom T, Skolnick P . Antidepressant-like actions of an AMPA receptor potentiator (LY392098). Neuropharmacology 2001; 40: 1028–1033.
Bai F, Bergeron M, Nelson DL . Chronic AMPA receptor potentiator (LY451646) treatment increases cell proliferation in adult rat hippocampus. Neuropharmacology 2003; 44: 1013–1021.
Skolnick P . Antidepressants for the new millennium. Eur J Pharmacol 1999; 375: 31–40.
Kroczka B, Branski P, Palucha A, Pilc A, Nowak G . Antidepressant-like properties of zinc in rodent forced swim test. Brain Res Bull 2001; 55: 297–300.
Layer RT, Popik P, Olds T, Skolnick P . Antidepressant-like actions of the polyamine site NMDA antagonist, eliprodil (SL-82.0715). Pharmacol Biochem Behav 1995; 52: 621–627.
Papp M, Moryl E . Antidepressant-like effects of 1-aminocyclopropanecarboxylic acid and D-cycloserine in an animal model of depression. Eur J Pharmacol 1996; 316: 145–151.
Przegalinski E, Tatarczynska E, Deren-Wesolek A, Chojnacka-Wojcik E . Antidepressant-like effects of a partial agonist at strychnine-insensitive glycine receptors and a competitive NMDA receptor antagonist. Neuropharmacology 1997; 36: 31–37.
Ossowska G, Klenk-Majewska B, Szymczyk G . The effect of NMDA antagonists on footshock-induced fighting behavior in chronically stressed rats. J Physiol Pharmacol 1997; 48: 127–135.
Panconi E, Roux J, Altenbaumer M, Hampe S, Porsolt RD . MK-801 and enantiomers: potential antidepressants or false positives in classical screening models? Pharmacol Biochem Behav 1993; 46: 15–20.
Maj J, Rogoz Z, Skuza G, Sowinska H . The effect of CGP 37849 and CGP 39551, competitive NMDA receptor antagonists, in the forced swimming test. Pol J Pharmacol Pharm 1992; 44: 337–346.
Maj J, Rogoz Z, Skuza G, Sowinska H . Effects of MK-801 and antidepressant drugs in the forced swimming test in rats. Eur Neuropsychopharmacol 1992; 2: 37–41.
Papp M, Moryl E . Antidepressant activity of non-competitive and competitive NMDA receptor antagonists in a chronic mild stress model of depression. Eur J Pharmacol 1994; 263: 1–7.
Papp M, Moryl E, Willner P . Pharmacological validation of the chronic mild stress model of depression. Eur J Pharmacol 1996; 296: 129–136.
Nowak G, Legutko B, Skolnick P, Popik P . Adaptation of cortical NMDA receptors by chronic treatment with specific serotonin reuptake inhibitors. Eur J Pharmacol 1998; 342: 367–370.
Nibuya M, Morinobu S, Duman RS . Regulation of BDNF and trkB mRNA in rat brain by chronic electroconvulsive seizure and antidepressant drug treatments. J Neurosci 1995; 15: 7539–7547.
Brandoli C, Sanna A, De Bernardi MA, Follesa P, Brooker G, Mocchetti I . Brain-derived neurotrophic factor and basic fibroblast growth factor downregulate NMDA receptor function in cerebellar granule cells. J Neurosci 1998; 18: 7953–7961.
Palucha A, Branski P, Szewczyk B, Wieronska JM, Klak K, Pilc A . Potential antidepressant-like effect of MTEP, a potent and highly selective mGluR5 antagonist. Pharmacol Biochem Behav 2005; 81: 901–906.
Awad H, Hubert GW, Smith Y, Levey AI, Conn PJ . Activation of metabotropic glutamate receptor 5 has direct excitatory effects and potentiates NMDA receptor currents in neurons of the subthalamic nucleus. J Neurosci 2000; 20: 7871–7879.
Zahorodna A, Bijak M . An antidepressant-induced decrease in the responsiveness of hippocampal neurons to group I metabotropic glutamate receptor activation. Eur J Pharmacol 1999; 386: 173–179.
Pilc A, Branski P, Palucha A, Tokarski K, Bijak M . Antidepressant treatment influences group I of glutamate metabotropic receptors in slices from hippocampal CA1 region. Eur J Pharmacol 1998; 349: 83–87.
Zarate CA, Quiroz J, Payne J, Manji HK . Modulators of the glutamatergic system: implications for the development of improved therapeutics in mood disorders. Psychopharmacol Bull 2002; 36: 35–83.
Krystal JH, Sanacora G, Blumberg H, Anand A, Charney DS, Marek G et al. Glutamate and GABA systems as targets for novel antidepressant and mood-stabilizing treatments. Mol Psychiatry 2002; 7 (Suppl 1): S71–80.
Auer DP, Putz B, Kraft E, Lipinski B, Schill J, Holsboer F . Reduced glutamate in the anterior cingulate cortex in depression: an in vivo proton magnetic resonance spectroscopy study. Biol Psychiatry 2000; 47: 305–313.
Boldrini M, Santiago AN, Hen R, Dwork AJ, Rosoklija GB, Tamir H et al. Hippocampal granule neuron number and dentate gyrus volume in antidepressant-treated and untreated major depression. Neuropsychopharmacology 2013; 38: 1068–1077.
Hasler G, Neumeister A, van der Veen JW, Tumonis T, Bain EE, Shen J et al. Normal prefrontal gamma-aminobutyric acid levels in remitted depressed subjects determined by proton magnetic resonance spectroscopy. Biol Psychiatry 2005; 58: 969–973.
Cryan JF, Kaupmann K . Don't worry 'B' happy!: a role for GABA(B) receptors in anxiety and depression. Trends Pharmacol Sci 2005; 26: 36–43.
Pilc A, Nowak G . GABAergic hypotheses of anxiety and depression: focus on GABA-B receptors. Drugs Today (Barc) 2005; 41: 755–766.
Streeter CC, Hennen J, Ke Y, Jensen JE, Sarid-Segal O, Nassar LE et al. Prefrontal GABA levels in cocaine-dependent subjects increase with pramipexole and venlafaxine treatment. Psychopharmacology 2005; 182: 516–526.
Sanacora G, Mason GF, Rothman DL, Krystal JH . Increased occipital cortex GABA concentrations in depressed patients after therapy with selective serotonin reuptake inhibitors. Am J Psychiatry 2002; 159: 663–665.
Bhagwagar Z, Wylezinska M, Taylor M, Jezzard P, Matthews PM, Cowen PJ . Increased brain GABA concentrations following acute administration of a selective serotonin reuptake inhibitor. Am J Psychiatry 2004; 161: 368–370.
Sanacora G, Mason GF, Rothman DL, Hyder F, Ciarcia JJ, Ostroff RB et al. Increased cortical GABA concentrations in depressed patients receiving ECT. Am J Psychiatry 2003; 160: 577–579.
Underwood MD, Kassir SA, Bakalian MJ, Galfalvy H, Mann JJ, Arango V . Neuron density and serotonin receptor binding in prefrontal cortex in suicide. Int J Neuropsychopharmacol 2012; 15: 435–447.
Benes FM, Majocha R, Bird ED, Marotta CA . Increased vertical axon numbers in cingulate cortex of schizophrenics. Arch Gen Psychiatry 1987; 44: 1017–1021.
Maciag D, Hughes J, O'Dwyer G, Pride Y, Stockmeier CA, Sanacora G et al. Reduced density of calbindin immunoreactive GABAergic neurons in the occipital cortex in major depression: relevance to neuroimaging studies. Bioll Psychiatry 2010; 67: 465–470.
Sequeira A, Mamdani F, Ernst C, Vawter MP, Bunney WE, Lebel V et al. Global brain gene expression analysis links glutamatergic and GABAergic alterations to suicide and major depression. PLoS One 2009; 4: e6585.
Petty F . GABA and mood disorders: a brief review and hypothesis. J Affect Disord 1995; 34: 275–281.
Lloyd KG, Morselli PL, Bartholini G . GABA and affective disorders. Mol Psychiatry 1987; 65: 159–165.
Shungu DC, Mao X, Gu M, Milak MS, Weiduscha N, Mayer D et al. ‘Glx’ measured by J-editing/MEGA-PRESS is primarily ‘pure’ glutamate…or is it? Proc Intl Soc Magn Reson Med 2013; 21: 1.
Carrier N, Kabbaj M . Sex differences in the antidepressant-like effects of ketamine. Neuropharmacology 2013; 70: 27–34.
Meyer JH, Wilson AA, Sagrati S, Hussey D, Carella A, Potter WZ et al. Serotonin transporter occupancy of five selective serotonin reuptake inhibitors at different doses: an [11C]DASB positron emission tomography study. Am J Psychiatry 2004; 161: 826–835.
Nair NP, Ahmed SK, Kin NM . Biochemistry and pharmacology of reversible inhibitors of MAO-A agents: focus on moclobemide. J Psychiatry Neurosci 1993; 18: 214–225.
Acknowledgements
This work was supported by a Brain and Behavior Research Foundation NARSAD Distinguished Investigator Award to Dr Mann and NIMH grants R01 MH-075895 to Dr Shungu and R01 MH-093637 to Dr Milak.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Dr Milak, Ms Proper, Ms Mulhern, Ms Parter, Dr Ogden, Dr Keilp, Ms Mao, Mr Cooper, Dr Shungu, Dr Rodriguez and Dr Suckow reported no biomedical financial interests or potential conflicts of interest. Dr Kegeles has received research grants from Pfizer and Amgen. Dr Oquendo receives royalties for use of the Columbia Suicide Severity Rating Scale and received financial compensation from Pfizer for the safety evaluation of a clinical facility, unrelated to this study. She has received unrestricted educational grants and/or lecture fees from Astra-Zeneca, Bristol Myers Squibb, Eli Lilly, Janssen, Otsuko, Pfizer, Sanofi-Aventis and Shire. Her family owns stock in Bristol Myers Squibb. Dr Mann received prior unrelated grants from Novartis and GSK and receives royalties for commercial use of the C-SSRS from the Research Foundation for Mental Health.
Rights and permissions
About this article
Cite this article
Milak, M., Proper, C., Mulhern, S. et al. A pilot in vivo proton magnetic resonance spectroscopy study of amino acid neurotransmitter response to ketamine treatment of major depressive disorder. Mol Psychiatry 21, 320–327 (2016). https://doi.org/10.1038/mp.2015.83
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/mp.2015.83
This article is cited by
-
Region- and time- specific effects of ketamine on cerebral blood flow: a randomized controlled trial
Neuropsychopharmacology (2023)
-
Connexin 43: insights into candidate pathological mechanisms of depression and its implications in antidepressant therapy
Acta Pharmacologica Sinica (2022)
-
Acute effects of ketamine on the pregenual anterior cingulate: linking spontaneous activation, functional connectivity, and glutamate metabolism
European Archives of Psychiatry and Clinical Neuroscience (2022)
-
Fine-tuning neural excitation/inhibition for tailored ketamine use in treatment-resistant depression
Translational Psychiatry (2021)
-
Ventromedial prefrontal cortex/anterior cingulate cortex Glx, glutamate, and GABA levels in medication-free major depressive disorder
Translational Psychiatry (2021)