Abstract
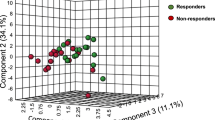
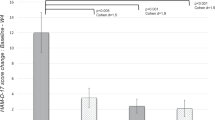
This study explores whether inflammatory biomarkers act as moderators of clinical response to omega-3 (n-3) fatty acids in subjects with major depressive disorder (MDD). One hundred fifty-five subjects with Diagnostic and Statistical Manual of Mental Disorders, 4th Edition (DSM-IV) MDD, a baseline 17-item Hamilton Depression Rating Scale (HAM-D-17) score ⩾15 and baseline biomarker data (interleukin (IL)-1ra, IL-6, high-sensitivity C-reactive protein (hs-CRP), leptin and adiponectin) were randomized between 18 May 2006 and 30 June 2011 to 8 weeks of double-blind treatment with eicosapentaenoic acid (EPA)-enriched n-3 1060 mg day−1, docosahexaenoic acid (DHA)-enriched n-3 900 mg day−1 or placebo. Outcomes were determined using mixed model repeated measures analysis for ‘high’ and ‘low’ inflammation groups based on individual and combined biomarkers. Results are presented in terms of standardized treatment effect size (ES) for change in HAM-D-17 from baseline to treatment week 8. Although overall treatment group differences were negligible (ES=−0.13 to +0.04), subjects with any ‘high’ inflammation improved more on EPA than placebo (ES=−0.39) or DHA (ES=−0.60) and less on DHA than placebo (ES=+0.21); furthermore, EPA-placebo separation increased with increasing numbers of markers of high inflammation. Subjects randomized to EPA with ‘high’ IL-1ra or hs-CRP or low adiponectin (‘high’ inflammation) had medium ES decreases in HAM-D-17 scores vs subjects ‘low’ on these biomarkers. Subjects with ‘high’ hs-CRP, IL-6 or leptin were less placebo-responsive than subjects with low levels of these biomarkers (medium to large ES differences). Employing multiple markers of inflammation facilitated identification of a more homogeneous cohort of subjects with MDD responding to EPA vs placebo in our cohort. Studies are needed to replicate and extend this proof-of-concept work.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Schmidt HD, Shelton RC, Duman RS . Functional biomarkers of depression: diagnosis, treatment, and pathophysiology. Neuropsychopharmacology 2011; 36: 2375–2394.
McNamara RK, Lotrich FE . Elevated immune-inflammatory signaling in mood disorders: a new therapeutic target? Expert Rev Neurother 2012; 12: 1143–1161.
Straub RH . Evolutionary medicine and chronic inflammatory state—known and new concepts in pathophysiology. J Mol Med (Berl) 2012; 90: 523–534.
Ridker PM . Inflammatory biomarkers and risks of myocardial infarction, stroke, diabetes, and total mortality: implications for longevity. Nutr Rev 2007; 65: S253–S259.
Miller AH, Maletic V, Raison CL . Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biol Psychiatry 2009; 65: 732–741.
Maes M, Ruckoanich P, Chang YS, Mahanonda N, Berk M . Multiple aberrations in shared inflammatory and oxidative & nitrosative stress (IO&NS) pathways explain the co-association of depression and cardiovascular disorder (CVD), and the increased risk for CVD and due mortality in depressed patients. Prog Neuropsychopharmacol Biol Psychiatry 2011; 35: 769–783.
Capuron L, Miller AH . Immune system to brain signaling: neuropsychopharmacological implications. Pharmacol Ther 2011; 130: 226–238.
Miller AH . Conceptual confluence: the kynurenine pathway as a common target for ketamine and the convergence of the inflammation and glutamate hypotheses of depression. Neuropsychopharmacology 2013; 38: 1607–1608.
Muller N, Schwarz MJ . The immune-mediated alteration of serotonin and glutamate: towards an integrated view of depression. Mol Psychiatry 2007; 12: 988–1000.
Steiner J, Walter M, Gos T, Guillemin GJ, Bernstein HG, Sarnyai Z et al. Severe depression is associated with increased microglial quinolinic acid in subregions of the anterior cingulate gyrus: evidence for an immune-modulated glutamatergic neurotransmission? J Neuroinflammation 2011; 8: 94.
Morris AA, Zhao L, Ahmed Y, Stoyanova N, De Staercke C, Hooper WC et al. Association between depression and inflammation—differences by race and sex: the META-Health study. Psychosom Med 2011; 73: 462–468.
Su KP, Lai HC, Yang HT, Su WP, Peng CY, Chang JP et al. Omega-3 fatty acids in the prevention of interferon-alpha-induced depression: results from a randomized, controlled trial. Biol Psychiatry 2014; 76: 559–566.
Musselman DL, Lawson DH, Gumnick JF, Manatunga AK, Penna S, Goodkin RS et al. Paroxetine for the prevention of depression induced by high-dose interferon alfa. N Engl J Med 2001; 344: 961–966.
Raison CL, Rutherford RE, Woolwine BJ, Shuo C, Schettler P, Drake DF et al. A randomized controlled trial of the tumor necrosis factor antagonist infliximab for treatment-resistant depression: the role of baseline inflammatory biomarkers. JAMA Psychiatry 2013; 70: 31–41.
Bang HO, Dyerberg J, Nielsen AB . Plasma lipid and lipoprotein pattern in Greenlandic West-coast Eskimos. Lancet 1971; 1: 1143–1145.
Hibbeln JR . Fish consumption and major depression. Lancet 1998; 351: 1213.
Rizos EC, Ntzani EE, Bika E, Kostapanos MS, Elisaf MS . Association between omega-3 fatty acid supplementation and risk of major cardiovascular disease events: a systematic review and meta-analysis. JAMA 2012; 308: 1024–1033.
Yates CM, Calder PC, Ed Rainger G . Pharmacology and therapeutics of omega-3 polyunsaturated fatty acids in chronic inflammatory disease. Pharmacol Ther 2014; 141: 272–282.
Gertsik L, Poland RE, Bresee C, Rapaport MH . Omega-3 fatty acid augmentation of citalopram treatment for patients with major depressive disorder. J Clin Psychopharmacol 2012; 32: 61–64.
Jazayeri S, Tehrani-Doost M, Keshavarz SA, Hosseini M, Djazayery A, Amini H et al. Comparison of therapeutic effects of omega-3 fatty acid eicosapentaenoic acid and fluoxetine, separately and in combination, in major depressive disorder. Aust N Z J Psychiatry 2008; 42: 192–198.
Lesperance F, Frasure-Smith N, St-Andre E, Turecki G, Lesperance P, Wisniewski SR . The efficacy of omega-3 supplementation for major depression: a randomized controlled trial. J Clin Psychiatry 2011; 72: 1054–1062.
Mischoulon D, Best-Popescu C, Laposata M, Merens W, Murakami JL, Wu SL et al. A double-blind dose-finding pilot study of docosahexaenoic acid (DHA) for major depressive disorder. Eur Neuropsychopharmacol 2008; 18: 639–645.
Marangell LB, Martinez JM, Zboyan HA, Kertz B, Kim HF, Puryear LJ . A double-blind, placebo-controlled study of the omega-3 fatty acid docosahexaenoic acid in the treatment of major depression. Am J Psychiatry 2003; 160: 996–998.
Mischoulon D, Nierenberg AA, Schettler PJ, Kinkead BL, Fehling K, Martinson MA et al. A double-blind, randomized controlled clinical trial comparing eicosapentaenoic acid versus docosahexaenoic acid for depression. J Clin Psychiatry 2014; 76: 54–61.
Bloch MH, Hannestad J . Omega-3 fatty acids for the treatment of depression: systematic review and meta-analysis. Mol Psychiatry 2012; 17: 1272–1282.
Lin PY, Su KP . A meta-analytic review of double-blind, placebo-controlled trials of antidepressant efficacy of omega-3 fatty acids. J Clin Psychiatry 2007; 68: 1056–1061.
Sublette ME, Ellis SP, Geant AL, Mann JJ . Meta-analysis of the effects of eicosapentaenoic acid (EPA) in clinical trials in depression. J Clin Psychiatry 2011; 72: 1577–1584.
Lin PY, Mischoulon D, Freeman MP, Matsuoka Y, Hibbeln J, Belmaker RH et al. Are omega-3 fatty acids antidepressants or just mood-improving agents? The effect depends upon diagnosis, supplement preparation, and severity of depression. Mol Psychiatry 2012; 17: 1161–1163.
Martins JG, Bentsen H, Puri BK . Eicosapentaenoic acid appears to be the key omega-3 fatty acid component associated with efficacy in major depressive disorder: a critique of Bloch and Hannestad and updated meta-analysis. Mol Psychiatry 2012; 17: 1144–1149.
Fenton JI, Hord NG, Ghosh S, Gurzell EA . Immunomodulation by dietary long chain omega-3 fatty acids and the potential for adverse health outcomes. Prostaglandins Leukot Essent Fatty Acids 2013; 89: 379–390.
Giordano E, Visioli F . Long-chain omega 3 fatty acids: molecular bases of potential antioxidant actions. Prostaglandins Leukot Essent Fatty Acids 2014; 90: 1–4.
First MB, Spitzer RL, Gibbon M, JBW Williams . Structured Clinical Interview for DSM-IV Axis I Disorders—Patient Edition (SCID-I/P, Version 2.0). Biometrics Research Department, New York State Psychiatric Institute: New York, 1995.
Hamilton M . Development of a rating scale for primary depressive illness. Br J Soc Clin Psychol 1967; 6: 278–296.
Pearson TA, Mensah GA, Alexander RW, Anderson JL, Cannon RO 3rd, Criqui M et al. Markers of inflammation and cardiovascular disease: application to clinical and public health practice: a statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation 2003; 107: 499–511.
Fantuzzi G . Adiponectin in inflammatory and immune-mediated diseases. Cytokine 2013; 64: 1–10.
Marques-Vidal P, Schmid R, Bochud M, Bastardot F, von Kanel R, Paccaud F et al. Adipocytokines, hepatic and inflammatory biomarkers and incidence of type 2 diabetes. the CoLaus study. PLoS One 2012; 7: e51768.
Freeman GH, Halton JH . Note on an exact treatment of contingency, goodness of fit and other problems of significance. Biometrika 1951; 38: 141–149.
Kohler O, Benros ME, Nordentoft M, Farkouh ME, Iyengar RL, Mors O et al. Effect of anti-inflammatory treatment on depression, depressive symptoms, and adverse effects: a systematic review and meta-analysis of randomized clinical trials. JAMA Psychiatry 2014; 71: 1381–1391.
Rakel DP . The anti-inflammatory diet. Integrative Medicine, 1st Edition. D.R. Saunders, Editor: Philadelphia, 2003.
Warner-Schmidt JL, Vanover KE, Chen EY, Marshall JJ, Greengard P . Antidepressant effects of selective serotonin reuptake inhibitors (SSRIs) are attenuated by antiinflammatory drugs in mice and humans. Proc Natl Acad Sci USA 2011; 108: 9262–9267.
Gueorguieva R, Mallinckrodt C, Krystal JH . Trajectories of depression severity in clinical trials of duloxetine: insights into antidepressant and placebo responses. Arch Gen Psychiatry 2011; 68: 1227–1237.
Capuron L, Su S, Miller AH, Bremner JD, Goldberg J, Vogt GJ et al. Depressive symptoms and metabolic syndrome: is inflammation the underlying link? Biol Psychiatry 2008; 64: 896–900.
Howren MB, Lamkin DM, Suls J . Associations of depression with C-reactive protein, IL-1, and IL-6: a meta-analysis. Psychosom Med 2009; 71: 171–186.
Lopresti AL, Drummond PD . Obesity and psychiatric disorders: commonalities in dysregulated biological pathways and their implications for treatment. Prog Neuropsychopharmacol Biol Psychiatry 2013; 45: 92–99.
Zeugmann S, Quante A, Heuser I, Schwarzer R, Anghelescu I . Inflammatory biomarkers in 70 depressed inpatients with and without the metabolic syndrome. J Clin Psychiatry 2010; 71: 1007–1016.
Hribal ML, Fiorentino TV, Sesti G . Role of C reactive protein (CRP) in leptin resistance. Curr Pharm Des 2014; 20: 609–615.
Acknowledgements
The study was funded by grant 5R01MH74085 from the National Institutes of Health to DM and MHR. EPA-enriched and DHA-enriched preparations and matching placebos were provided by Nordic Naturals (Watsonville, CA).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
DM has received research support from the Bowman Family Foundation, Fisher Wallace, Nordic Naturals, Methylation Sciences (MSI) and PharmoRx. He has received honoraria for speaking from Pamlab, and the Massachusetts General Hospital Psychiatry Academy. He has received royalties from Lippincott Williams & Wilkins for the book 'Natural Medications for Psychiatric Disorders: Considering the Alternatives'. AAN has served as a consultant to: Appliance Computing (Mindsite), Brain Cells, Brandeis University, Bristol-Myers Squibb, Clintara, Dainippon Sumitomo (now Sunovion), Eli Lilly and Company, EpiQ, Forest, Novartis, PamLabs, PGx Health, Shire, Schering-Plough, Sunovion, Takeda Pharmaceuticals, Teva and Targacept. He has consulted through the MGH Clinical Trials Network and Institute (CTNI): Astra Zeneca, Brain Cells, Dainippon Sumitomo/Sepracor, Johnson and Johnson, Labopharm, Merck, Methylation Sciences, Novartis, PGx Health, Shire, Schering-Plough, Targacept and Takeda/Lundbeck Pharmaceuticals. AAN received honoraria or travel expenses including CME activities from: APSARD, Belvoir Publishing, Boston Center for the Arts, University of Texas Southwestern Dallas, Hillside Hospital, American Drug Utilization Review, American Society for Clinical Psychopharmacology, Bayamon Region Psychiatric Society, San Juan, PR, Baystate Medical Center, Canadian Psychiatric Association, Columbia University, Douglas Hospital/McGill University, IMEDEX, International Society for Bipolar Disorders, Israel Society for Biological Psychiatry, John Hopkins University, MJ Consulting, New York State, Massachusetts Association of College Counselors, Medscape, MBL Publishing, Physicians Postgraduate Press, Ryan Licht Sang Foundation, Slack Publishing, SUNY Buffalo, University of Florida, University of Miami, University of Wisconsin, University of Pisa and SciMed. AAN is a presenter for the Massachusetts General Hospital Psychiatry Academy (MGHPA). The education programs conducted by the MGHPA were supported through Independent Medical Education (IME) grants from the following pharmaceutical companies in 2008: Astra Zeneca, Eli Lilly and Janssen Pharmaceuticals; in 2009 Astra Zeneca, Eli Lilly and Bristol-Myers Squibb. No speaker bureaus or boards since 2003. AAN owns stock options in Appliance Computing and Brain Cells. Additional income is possible from Infomedic.com depending on overall revenues of the company, but no revenue has been received to date. Through MGH, AAN is named for copyrights to: the Clinical Positive Affect Scale and the MGH Structured Clinical Interview for the Montgomery-Asberg Depression Scale exclusively licensed to the MGH Clinical Trials Network and Institute (CTNI). AAN has received grant/research support through MGH from AHRQ, Cephalon, Forest, Mylan, NIMH, PamLabs, Pfizer Pharmaceuticals, Takeda, Elan and Shire. PJS works part-time both as Senior Research Associate in the Department of Psychiatry and Behavioral Sciences at the Emory University School of Medicine, Atlanta, Georgia; as well as Principal Statistician in the Department of Psychiatry of the School of Medicine at the University of California, San Diego. She has no other direct or indirect affiliations or financial interests in connection with the contents of this paper. MHR has provided consulting services to PAX (unpaid) and has been funded by the NIH. The remaining authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Molecular Psychiatry website
Supplementary information
PowerPoint slides
Rights and permissions
About this article
Cite this article
Rapaport, M., Nierenberg, A., Schettler, P. et al. Inflammation as a predictive biomarker for response to omega-3 fatty acids in major depressive disorder: a proof-of-concept study. Mol Psychiatry 21, 71–79 (2016). https://doi.org/10.1038/mp.2015.22
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/mp.2015.22
This article is cited by
-
Omega-3 fatty acids as adjuvant therapy in the adverse effects of antineoplastic treatment for breast cancer: a systematic review
Nutrire (2023)
-
Clinical response to EPA supplementation in patients with major depressive disorder is associated with higher plasma concentrations of pro-resolving lipid mediators
Neuropsychopharmacology (2023)
-
Efficacy of eicosapentaenoic acid in inflammatory depression: study protocol for a match-mismatch trial
BMC Psychiatry (2022)
-
n-3 PUFAs for depression: treatment effect or absence-of-placebo effect?
Translational Psychiatry (2022)
-
Reduced erythrocyte membrane polyunsaturated fatty acid levels indicate diminished treatment response in patients with multi- versus first-episode schizophrenia
Schizophrenia (2022)