Abstract
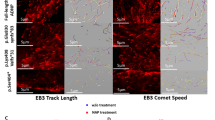
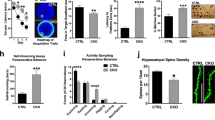
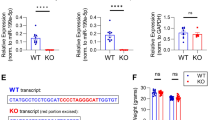
The NAP motif of activity-dependent neuroprotective protein (ADNP) enhanced memory scores in patients suffering from mild cognitive impairment and protected activities of daily living in schizophrenia patients, while fortifying microtubule (MT)-dependent axonal transport, in mice and flies. The question is how does NAP fortify MTs? Our sequence analysis identified the MT end-binding protein (EB1)-interacting motif SxIP (SIP, Ser-Ile-Pro) in ADNP/NAP and showed specific SxIP binding sites in all members of the EB protein family (EB1–3). Others found that EB1 enhancement of neurite outgrowth is attenuated by EB2, while EB3 interacts with postsynaptic density protein 95 (PSD-95) to modulate dendritic plasticity. Here, NAP increased PSD-95 expression in dendritic spines, which was inhibited by EB3 silencing. EB1 or EB3, but not EB2 silencing inhibited NAP-mediated cell protection, which reflected NAP binding specificity. NAPVSKIPQ (SxIP=SKIP), but not NAPVAAAAQ mimicked NAP activity. ADNP, essential for neuronal differentiation and brain formation in mouse, a member of the SWI/SNF chromatin remodeling complex and a major protein mutated in autism and deregulated in schizophrenia in men, showed similar EB interactions, which were enhanced by NAP treatment. The newly identified shared MT target of NAP/ADNP is directly implicated in synaptic plasticity, explaining the breadth and efficiency of neuroprotective/neurotrophic capacities.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Bassan M, Zamostiano R, Davidson A, Pinhasov A, Giladi E, Perl O et al. Complete sequence of a novel protein containing a femtomolar-activity-dependent neuroprotective peptide. J Neurochem 1999; 72: 1283–1293.
Zamostiano R, Pinhasov A, Gelber E, Steingart RA, Seroussi E, Giladi E et al. Cloning and characterization of the human activity-dependent neuroprotective protein. J Biol Chem 2001; 276: 708–714.
Helsmoortel C, Vulto-van Silfhout AT, Coe BP, Vandeweyer G, Rooms L, van den Ende J et al. A SWI/SNF-related autism syndrome caused by de novo mutations in ADNP. Nat Genet 2014; 46: 380–384.
Dresner E, Agam G, Gozes I . Activity-dependent neuroprotective protein (ADNP) expression level is correlated with the expression of the sister protein ADNP2: deregulation in schizophrenia. Eur Neuropsychopharmacol 2011; 21: 355–361.
Merenlender-Wagner A, Malishkevich A, Shemer Z, Udawela M, Gibbons A, Scarr E et al. Autophagy has a key role in the pathophysiology of schizophrenia. Mol Psychiatry advance online publication, 24 December 2013; doi:10.1038/mp.2013.174.
Schirer Y, Malishkevich A, Ophir Y, Lewis J, Giladi E, Gozes I . Novel marker for the onset of frontotemporal dementia: early increase in activity-dependent neuroprotective protein (ADNP) in the face of tau mutation. PLoS ONE 2014; 9: e87383.
Yang MH, Yang YH, Lu CY, Jong SB, Chen LJ, Lin YF et al. Activity-dependent neuroprotector homeobox protein: A candidate protein identified in serum as diagnostic biomarker for Alzheimer's disease. J Proteomics 2012; 75: 3617–3629.
Mandel S, Gozes I . Activity-dependent neuroprotective protein constitutes a novel element in the SWI/SNF chromatin remodeling complex. J Biol Chem 2007; 282: 34448–34456.
Mosch K, Franz H, Soeroes S, Singh PB, Fischle W . HP1 recruits activity-dependent neuroprotective protein to H3K9me3 marked pericentromeric heterochromatin for silencing of major satellite repeats. PLoS ONE 2011; 6: e15894.
Pinhasov A, Mandel S, Torchinsky A, Giladi E, Pittel Z, Goldsweig AM et al. Activity-dependent neuroprotective protein: a novel gene essential for brain formation. Brain Res Dev Brain Res 2003; 144: 83–90.
Mandel S, Rechavi G, Gozes I . Activity-dependent neuroprotective protein (ADNP) differentially interacts with chromatin to regulate genes essential for embryogenesis. Dev Biol 2007; 303: 814–824.
Dresner E, Malishkevich A, Arviv C, Leibman Barak S, Alon S, Ofir R et al. Novel evolutionary-conserved role for the activity-dependent neuroprotective protein (ADNP) family that is important for erythropoiesis. J Biol Chem 2012; 287: 40173–40185.
Furman S, Steingart RA, Mandel S, Hauser JM, Brenneman DE, Gozes I . Subcellular localization and secretion of activity-dependent neuroprotective protein in astrocytes. Neuron Glia Biol 2004; 1: 193–199.
Mandel S, Spivak-Pohis I, Gozes I . ADNP differential nucleus/cytoplasm localization in neurons suggests multiple roles in neuronal differentiation and maintenance. J Mol Neurosci 2008; 35: 127–141.
Gennet N, Herden C, Bubb VJ, Quinn JP, Kipar A . Expression of activity-dependent neuroprotective protein in the brain of adult rats. Histol Histopathol 2008; 23: 309–317.
Verhey KJ, Gaertig J . The tubulin code. Cell Cycle 2007; 6: 2152–2160.
Baas PW, Ahmad FJ . Beyond taxol: microtubule-based treatment of disease and injury of the nervous system. Brain 2013; 136: 2937–2951.
Sayas CL, Avila J . Regulation of EB1/3 proteins by classical MAPs in neurons. Bioarchitecture 2014; 4: 1–5.
Su LK, Qi Y . Characterization of human MAPRE genes and their proteins. Genomics 2001; 71: 142–149.
Nakagawa H, Koyama K, Murata Y, Morito M, Akiyama T, Nakamura Y . EB3, a novel member of the EB1 family preferentially expressed in the central nervous system, binds to a CNS-specific APC homologue. Oncogene 2000; 19: 210–216.
Stepanova T, Slemmer J, Hoogenraad CC, Lansbergen G, Dortland B, De Zeeuw CI et al. Visualization of microtubule growth in cultured neurons via the use of EB3-GFP (end-binding protein 3-green fluorescent protein). J Neurosci 2003; 23: 2655–2664.
Geraldo S, Khanzada UK, Parsons M, Chilton JK, Gordon-Weeks PR . Targeting of the F-actin-binding protein drebrin by the microtubule plus-tip protein EB3 is required for neuritogenesis. Nat Cell Biol 2008; 10: 1181–1189.
Gu C, Zhou W, Puthenveedu MA, Xu M, Jan YN, Jan LY . The microtubule plus-end tracking protein EB1 is required for Kv1 voltage-gated K+ channel axonal targeting. Neuron 2006; 52: 803–816.
Jaworski J, Kapitein LC, Gouveia SM, Dortland BR, Wulf PS, Grigoriev I et al. Dynamic microtubules regulate dendritic spine morphology and synaptic plasticity. Neuron 2009; 61: 85–100.
Komarova Y, Lansbergen G, Galjart N, Grosveld F, Borisy GG, Akhmanova A . EB1 and EB3 control CLIP dissociation from the ends of growing microtubules. Mol Biol Cell 2005; 16: 5334–5345.
Laketa V, Simpson JC, Bechtel S, Wiemann S, Pepperkok R . High-content microscopy identifies new neurite outgrowth regulators. Mol Biol Cell 2007; 18: 242–252.
Arens J, Duong TT, Dehmelt L . A morphometric screen identifies specific roles for microtubule-regulating genes in neuronal development of P19 stem cells. PLoS ONE 2013; 8: e79796.
Sen I, Veprintsev D, Akhmanova A, Steinmetz MO . End binding proteins are obligatory dimers. PLoS ONE 2013; 8: e74448.
De Groot CO, Jelesarov I, Damberger FF, Bjelic S, Scharer MA, Bhavesh NS et al. Molecular insights into mammalian end-binding protein heterodimerization. J Biol Chem 2010; 285: 5802–5814.
Honnappa S, Gouveia SM, Weisbrich A, Damberger FF, Bhavesh NS, Jawhari H et al. An EB1-binding motif acts as a microtubule tip localization signal. Cell 2009; 138: 366–376.
Laht P, Pill K, Haller E, Veske A . Plexin-B3 interacts with EB-family proteins through a conserved motif. Biochim Biophys Acta 2012; 1820: 888–893.
Tomasoni R, Repetto D, Morini R, Elia C, Gardoni F, Di Luca M et al. SNAP-25 regulates spine formation through postsynaptic binding to p140Cap. Nat Commun 2013; 4: 2136.
Matsuoka Y, Jouroukhin Y, Gray AJ, Ma L, Hirata-Fukae C, Li HF et al. A neuronal microtubule-interacting agent, NAPVSIPQ, reduces tau pathology and enhances cognitive function in a mouse model of Alzheimer's disease. J Pharmacol Exp Ther 2008; 325: 146–153.
Shiryaev N, Jouroukhin Y, Giladi E, Polyzoidou E, Grigoriadis NC, Rosenmann H et al. NAP protects memory, increases soluble tau and reduces tau hyperphosphorylation in a tauopathy model. Neurobiol Dis 2009; 34: 381–388.
Vulih-Shultzman I, Pinhasov A, Mandel S, Grigoriadis N, Touloumi O, Pittel Z et al. Activity-dependent neuroprotective protein snippet NAP reduces tau hyperphosphorylation and enhances learning in a novel transgenic mouse model. J Pharmacol Exp Ther 2007; 323: 438–449.
Oz S, Ivashko-Pachima Y, Gozes I . The ADNP derived peptide, NAP modulates the tubulin pool: implication for neurotrophic and neuroprotective activities. PLoS One 2012; 7: e51458.
Sudo H, Baas PW . Strategies for diminishing katanin-based loss of microtubules in tauopathic neurodegenerative diseases. Hum Mol Genet 2011; 20: 763–778.
Jouroukhin Y, Ostritsky R, Assaf Y, Pelled G, Giladi E, Gozes I . NAP (davunetide) modifies disease progression in a mouse model of severe neurodegeneration: Protection against impairments in axonal transport. Neurobiol Dis 2013; 56: 79–94.
Quraishe S, Cowan CM, Mudher A . NAP (davunetide) rescues neuronal dysfunction in a Drosophila model of tauopathy. Mol Psychiatry 2013; 18: 834–842.
Gozes I, Stewart A, Morimoto B, Fox A, Sutherland K, Schmechel D . Addressing Alzheimer's disease tangles: from NAP to AL-108. Curr Alzheimer Res 2009; 6: 455–460.
Javitt DC, Buchanan RW, Keefe RS, Kern R, McMahon RP, Green MF et al. Effect of the neuroprotective peptide davunetide (AL-108) on cognition and functional capacity in schizophrenia. Schizophr Res 2012; 136: 25–31.
Jarskog LF, Dong Z, Kangarlu A, Colibazzi T, Girgis RR, Kegeles LS et al. Effects of davunetide on N-acetylaspartate and choline in dorsolateral prefrontal cortex in patients with schizophrenia. Neuropsychopharmacology 2013; 38: 1245–1252.
Divinski I, Mittelman L, Gozes I . A femtomolar acting octapeptide interacts with tubulin and protects astrocytes against zinc intoxication. J Biol Chem 2004; 279: 28531–28538.
Divinski I, Holtser-Cochav M, Vulih-Schultzman I, Steingart RA, Gozes I . Peptide neuroprotection through specific interaction with brain tubulin. J Neurochem 2006; 98: 973–984.
Gozes I, Barnstable CJ . Monoclonal antibodies that recognize discrete forms of tubulin. Proc Natl Acad Sci U S A 1982; 79: 2579–2583.
Gu J, Firestein BL, Zheng JQ . Microtubules in dendritic spine development. J Neurosci 2008; 28: 12120–12124.
Alvarez VA, Sabatini BL . Anatomical and physiological plasticity of dendritic spines. Annu Rev Neurosci 2007; 30: 79–97.
Manzerra P, Behrens MM, Canzoniero LM, Wang XQ, Heidinger V, Ichinose T et al. Zinc induces a Src family kinase-mediated up-regulation of NMDA receptor activity and excitotoxicity. Proc Natl Acad Sci USA 2001; 98: 11055–11061.
Chen S, Charness ME . Ethanol inhibits neuronal differentiation by disrupting activity-dependent neuroprotective protein signaling. Proc Natl Acad Sci USA 2008; 105: 19962–19967.
Steingart RA, Gozes I . Recombinant activity-dependent neuroprotective protein protects cells against oxidative stress. Mol Cell Endocrinol 2006; 252: 148–153.
Esteves AR, Gozes I, Cardoso SM . The rescue of microtubule-dependent traffic recovers mitochondrial function in Parkinson's disease. Biochim Biophys Acta 2014; 1842: 7–21.
Lopus M, Manatschal C, Buey RM, Bjelic S, Miller HP, Steinmetz MO et al. Cooperative stabilization of microtubule dynamics by EB1 and CLIP-170 involves displacement of stably-bound Pi at microtubule ends. Biochemistry 2012; 51: 3021–3030.
Ito H, Atsuzawa K, Sudo K, Di Stefano P, Iwamoto I, Morishita R et al. Characterization of a multidomain adaptor protein, p140Cap, as part of a pre-synaptic complex. J Neurochem 2008; 107: 61–72.
Pascual M, Guerri C . The peptide NAP promotes neuronal growth and differentiation through extracellular signal-regulated protein kinase and Akt pathways, and protects neurons co-cultured with astrocytes damaged by ethanol. J Neurochem 2007; 103: 557–568.
Idan-Feldman A, Schirer Y, Polyzoidou E, Touloumi O, Lagoudaki R, Grigoriadis NC et al. Davunetide (NAP) as a preventative treatment for central nervous system complications in a diabetes rat model. Neurobiol Dis 2011; 44: 327–339.
Wilkemeyer MF, Chen SY, Menkari CE, Brenneman DE, Sulik KK, Charness ME . Differential effects of ethanol antagonism and neuroprotection in peptide fragment NAPVSIPQ prevention of ethanol-induced developmental toxicity. Proc Natl Acad Sci USA 2003; 100: 8543–8548.
Fishbein I, Segal M . Miniature synaptic currents become neurotoxic to chronically silenced neurons. Cereb Cortex 2007; 17: 1292–1306.
Sweet ES, Previtera ML, Fernandez JR, Charych EI, Tseng CY, Kwon M et al. PSD-95 alters microtubule dynamics via an association with EB3. J Neurosci 2011; 31: 1038–1047.
Kapitein LC, Yau KW, Gouveia SM, van der Zwan WA, Wulf PS, Keijzer N et al. NMDA receptor activation suppresses microtubule growth and spine entry. J Neurosci 2011; 31: 8194–8209.
Smith-Swintosky VL, Gozes I, Brenneman DE, D'Andrea MR, Plata-Salaman CR . Activity-dependent neurotrophic factor-9 and NAP promote neurite outgrowth in rat hippocampal and cortical cultures. J Mol Neurosci 2005; 25: 225–238.
Bhaskar K, Hobbs GA, Yen SH, Lee G . Tyrosine phosphorylation of tau accompanies disease progression in transgenic mouse models of tauopathy. Neuropathol Appl Neurobiol 2010; 36: 462–477.
Sala C, Sheng M . The fyn art of N-methyl-D-aspartate receptor phosphorylation. Proc Natl Acad Sci USA 1999; 96: 335–337.
Trepanier CH, Jackson MF, MacDonald JF . Regulation of NMDA receptors by the tyrosine kinase Fyn. FEBS J 2012; 279: 12–19.
Groveman BR, Feng S, Fang XQ, Pflueger M, Lin SX, Bienkiewicz EA et al. The regulation of N-methyl-D-aspartate receptors by Src kinase. FEBS J 2012; 279: 20–28.
Lee G, Newman ST, Gard DL, Band H, Panchamoorthy G . Tau interacts with src-family non-receptor tyrosine kinases. J Cell Sci 1998; 111: 3167–3177.
Ittner LM, Ke YD, Delerue F, Bi M, Gladbach A, van Eersel J et al. Dendritic function of tau mediates amyloid-beta toxicity in Alzheimer's disease mouse models. Cell 2010; 142: 387–397.
Babus LW, Little EM, Keenoy KE, Minami SS, Chen E, Song JM et al. Decreased dendritic spine density and abnormal spine morphology in Fyn knockout mice. Brain Res Dev Brain Res 2011; 1415: 96–102.
Sudhof TC, Malenka RC . Understanding synapses: past, present, and future. Neuron 2008; 60: 469–476.
Cull-Candy S, Brickley S, Farrant M . NMDA receptor subunits: diversity, development and disease. Curr Opin Neurobiol 2001; 11: 327–335.
Salter MW, Pitcher GM . Dysregulated Src upregulation of NMDA receptor activity: a common link in chronic pain and schizophrenia. FEBS J 2012; 279: 2–11.
Gozes I, Divinsky I, Pilzer I, Fridkin M, Brenneman DE, Spier AD . From vasoactive intestinal peptide (VIP) through activity-dependent neuroprotective protein (ADNP) to NAP: a view of neuroprotection and cell division. J Mol Neurosci 2003; 20: 315–322.
Alves-Silva J, Sanchez-Soriano N, Beaven R, Klein M, Parkin J, Millard TH et al. Spectraplakins promote microtubule-mediated axonal growth by functioning as structural microtubule-associated proteins and EB1-dependent +TIPs (tip interacting proteins). J Neurosci 2012; 32: 9143–9158.
Shim KS, Lubec G . Drebrin, a dendritic spine protein, is manifold decreased in brains of patients with Alzheimer's disease and Down syndrome. Neurosci Lett 2002; 324: 209–212.
Busciglio J, Pelsman A, Helguera P, Ashur-Fabian O, Pinhasov A, Brenneman DE et al. NAP and ADNF-9 protect normal and Down's syndrome cortical neurons from oxidative damage and apoptosis. Curr Pharm Des 2007; 13: 1091–1098.
Ohnuma T, Kato H, Arai H, Faull RL, McKenna PJ, Emson PC . Gene expression of PSD95 in prefrontal cortex and hippocampus in schizophrenia. Neuroreport 2000; 11: 3133–3137.
Acknowledgements
We thank Dr Shmuel Mandel for his work on drebrin identification. Initial studies were supported by Allon Therapeutics Inc., Joe and Grace Alter, Barbara and Don Seal, and the Oberfeld family. Further studies were supported by AMN Foundation and the Adams family (Montreal Circle of Friends of Tel Aviv University). Y Ivashco-Pachima is supported by the Joseph Sagol Scholarship for Brain Studies. These studies are in partial fulfilment of the requirements for graduate work at the Dr. Miriam and Sheldon G. Adelson Graduate School of Medicine, and at the Sackler Faculty of Medicine at Tel Aviv University of Drs. S. Oz and N. Skalka, Y. and O Kapitansky (M.Sc.), Y Ivashco-Pachima (Ph.D. studies) and A Malishkevich (Ph.D. studies). Professor Gozes is the incumbent of the Lily and Avraham Gildor Chair for the Investigation of Growth Factors and the Head of the Adams Super Center for Brain Studies, the Levie-Edersheim Gitter fMRI Institute and the Elton Laboratory for Molecular Neuroendocrinology. Professor Gozes is currently a Humboldt Award Recipient and a fellow at the Hanse-Wissenschftenkolleg, Germany.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Material accompanies the paper on the Molecular Psychiatry website
Supplementary information
Rights and permissions
About this article
Cite this article
Oz, S., Kapitansky, O., Ivashco-Pachima, Y. et al. The NAP motif of activity-dependent neuroprotective protein (ADNP) regulates dendritic spines through microtubule end binding proteins. Mol Psychiatry 19, 1115–1124 (2014). https://doi.org/10.1038/mp.2014.97
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/mp.2014.97
This article is cited by
-
The cytoplasmic localization of ADNP through 14-3-3 promotes sex-dependent neuronal morphogenesis, cortical connectivity, and calcium signaling
Molecular Psychiatry (2023)
-
Adnp-mutant mice with cognitive inflexibility, CaMKIIα hyperactivity, and synaptic plasticity deficits
Molecular Psychiatry (2023)
-
Unexpected gender differences in progressive supranuclear palsy reveal efficacy for davunetide in women
Translational Psychiatry (2023)
-
Introducing ADNP and SIRT1 as new partners regulating microtubules and histone methylation
Molecular Psychiatry (2021)
-
Discovery of autism/intellectual disability somatic mutations in Alzheimer's brains: mutated ADNP cytoskeletal impairments and repair as a case study
Molecular Psychiatry (2021)