Abstract
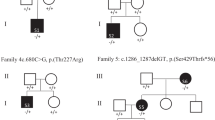
Autism spectrum disorders (ASDs) have been suggested to arise from abnormalities in the canonical and non-canonical Wnt signaling pathways. However, a direct connection between a human variant in a Wnt pathway gene and ASD-relevant brain pathology has not been established. Prickle2 (Pk2) is a post-synaptic non-canonical Wnt signaling protein shown to interact with post-synaptic density 95 (PSD-95). Here, we show that mice with disruption in Prickle2 display behavioral abnormalities including altered social interaction, learning abnormalities and behavioral inflexibility. Prickle2 disruption in mouse hippocampal neurons led to reductions in dendrite branching, synapse number and PSD size. Consistent with these findings, Prickle2 null neurons show decreased frequency and size of spontaneous miniature synaptic currents. These behavioral and physiological abnormalities in Prickle2 disrupted mice are consistent with ASD-like phenotypes present in other mouse models of ASDs. In 384 individuals with autism, we identified two with distinct, heterozygous, rare, non-synonymous PRICKLE2 variants (p.E8Q and p.V153I) that were shared by their affected siblings and inherited paternally. Unlike wild-type PRICKLE2, the PRICKLE2 variants found in ASD patients exhibit deficits in morphological and electrophysiological assays. These data suggest that these PRICKLE2 variants cause a critical loss of PRICKLE2 function. The data presented here provide new insight into the biological roles of Prickle2, its behavioral importance, and suggest disruptions in non-canonical Wnt genes such as PRICKLE2 may contribute to synaptic abnormalities underlying ASDs.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Geschwind D . Autism: searching for coherence. Biol Psychiatry 2007; 62: 949–950.
Geschwind DH, Levitt P . Autism spectrum disorders: developmental disconnection syndromes. Curr Opin Neurobiol 2007; 17: 103–111.
Persico AM, Bourgeron T . Searching for ways out of the autism maze: genetic, epigenetic and environmental clues. Trends Neurosci 2006; 29: 349–358.
Autism, Developmental Disabilities Monitoring Network Surveillance Year Principal I. Prevalence of autism spectrum disorders - autism and developmental disabilities monitoring network, 14 sites, United States, 2008. MMWR Surveill Summ 2012; 61: 1–19.
Canitano R . Epilepsy in autism spectrum disorders. Eur Child Adolesc Psychiatry 2007; 16: 61–66.
Fassio A, Patry L, Congia S, Onofri F, Piton A, Gauthier J et al. SYN1 loss-of-function mutations in autism and partial epilepsy cause impaired synaptic function. Hum Mol Genet 2011; 20: 2297–2307.
Bourgeron T . A synaptic trek to autism. Curr Opin Neurobiol 2009; 19: 231–234.
Bozzi Y, Casarosa S, Caleo M . Epilepsy as a neurodevelopmental disorder. Front Psychiatry 2012; 3: 19.
Schmeisser MJ, Ey E, Wegener S, Bockmann J, Stempel AV, Kuebler A et al. Autistic-like behaviours and hyperactivity in mice lacking ProSAP1/Shank2. Nature 2012; 486: 256–260.
Cuitino L, Godoy JA, Farias GG, Couve A, Bonansco C, Fuenzalida M et al. Wnt-5a modulates recycling of functional GABAA receptors on hippocampal neurons. J Neurosci 2010; 30: 8411–8420.
Farias GG, Alfaro IE, Cerpa W, Grabowski CP, Godoy JA, Bonansco C et al. Wnt-5a/JNK signaling promotes the clustering of PSD-95 in hippocampal neurons. J Biol Chem 2009; 284: 15857–15866.
Freese JL, Pino D, Pleasure SJ . Wnt signaling in development and disease. Neurobiol Dis 2010; 38: 148–153.
Rosso SB, Sussman D, Wynshaw-Boris A, Salinas PC . Wnt signaling through Dishevelled, Rac and JNK regulates dendritic development. Nat Neurosci 2005; 8: 34–42.
Varela-Nallar L, Alfaro IE, Serrano FG, Parodi J, Inestrosa NC . Wingless-type family member 5A (Wnt-5a) stimulates synaptic differentiation and function of glutamatergic synapses. Proc Natl Acad Sci USA. 2010; 107: 21164–21169.
Fujimura L, Watanabe-Takano H, Sato Y, Tokuhisa T, Hatano M . Prickle promotes neurite outgrowth via the Dishevelled dependent pathway in C1300 cells. Neurosci Lett 2009; 467: 6–10.
Hida Y, Fukaya M, Hagiwara A, Deguchi-Tawarada M, Yoshioka T, Kitajima I et al. Prickle2 is localized in the postsynaptic density and interacts with PSD-95 and NMDA receptors in the brain. J Biochem 2011; 149: 693–700.
Okuda H, Miyata S, Mori Y, Tohyama M . Mouse Prickle1 and Prickle2 are expressed in postmitotic neurons and promote neurite outgrowth. FEBS Lett 2007; 581: 4754–4760.
Tao H, Manak JR, Sowers L, Mei X, Kiyonari H, Abe T et al. Mutations in prickle orthologs cause seizures in flies, mice, and humans. Am J Hum Genet 2011; 88: 138–149.
Li F, Chong ZZ, Maiese K . Vital elements of the Wnt-Frizzled signaling pathway in the nervous system. Curr Neurovasc Res 2005; 2: 331–340.
Tree DR, Shulman JM, Rousset R, Scott MP, Gubb D, Axelrod JD . Prickle mediates feedback amplification to generate asymmetric planar cell polarity signaling. Cell 2002; 109: 371–381.
Moreau MM, Piguel N, Papouin T, Koehl M, Durand CM, Rubio ME et al. The planar polarity protein Scribble1 is essential for neuronal plasticity and brain function. J Neurosci 2010; 30: 9738–9752.
Long JM, LaPorte P, Paylor R, Wynshaw-Boris A . Expanded characterization of the social interaction abnormalities in mice lacking Dvl1. Genes Brain Behav 2004; 3: 51–62.
Okerlund ND, Kivimae S, Tong CK, Peng IF, Ullian EM, Cheyette BN . Dact1 is a postsynaptic protein required for dendrite, spine, and excitatory synapse development in the mouse forebrain. J Neurosci 2010; 30: 4362–4368.
Lijam N, Paylor R, McDonald MP, Crawley JN, Deng CX, Herrup K et al. Social interaction and sensorimotor gating abnormalities in mice lacking Dvl1. Cell 1997; 90: 895–905.
O'Connor N, Hermelin B . The memory structure of autistic idiot-savant mnemonists. Br J Psychol 1989; 80 (Pt 1): 97–111.
Wassink TH, Piven J, Vieland VJ, Huang J, Swiderski RE, Pietila J et al. Evidence supporting WNT2 as an autism susceptibility gene. Am J Med Genet 2001; 105: 406–413.
Gilman SR, Iossifov I, Levy D, Ronemus M, Wigler M, Vitkup D . Rare de novo variants associated with autism implicate a large functional network of genes involved in formation and function of synapses. Neuron 2011; 70: 898–907.
O'Roak BJ, Vives L, Girirajan S, Karakoc E, Krumm N, Coe BP et al. Sporadic autism exomes reveal a highly interconnected protein network of de novo mutations. Nature 2012; 485: 246–250.
Katoh M . Identification and characterization of human PRICKLE1 and PRICKLE2 genes as well as mouse Prickle1 and Prickle2 genes homologous to Drosophila tissue polarity gene prickle. Int J Mol Med 2003; 11: 249–256.
Narimatsu M, Bose R, Pye M, Zhang L, Miller B, Ching P et al. Regulation of planar cell polarity by Smurf ubiquitin ligases. Cell 2009; 137: 295–307.
McNamara JO . Cellular and molecular basis of epilepsy. J Neurosci 1994; 14: 3413–3425.
Auerbach BD, Osterweil EK, Bear MF . Mutations causing syndromic autism define an axis of synaptic pathophysiology. Nature 2011; 480: 63–68.
Yun SH, Trommer BL . Fragile X mice: reduced long-term potentiation and N-Methyl-D-Aspartate receptor-mediated neurotransmission in dentate gyrus. J Neurosci Res 2011; 89: 176–182.
Murata T, Furushima K, Hirano M, Kiyonari H, Nakamura M, Suda Y et al. ang is a novel gene expressed in early neuroectoderm, but its null mutant exhibits no obvious phenotype. Gene Expr Patterns 2004; 5: 171–178.
Yagi T, Tokunaga T, Furuta Y, Nada S, Yoshida M, Tsukada T et al. A novel ES cell line, TT2, with high germline-differentiating potency. Anal Biochem 1993; 214: 70–76.
Coryell MW, Ziemann AE, Westmoreland PJ, Haenfler JM, Kurjakovic Z, Zha XM et al. Targeting ASIC1a reduces innate fear and alters neuronal activity in the fear circuit. Biol Psychiatry 2007; 62: 1140–1148.
Sfakianos MK, Eisman A, Gourley SL, Bradley WD, Scheetz AJ, Settleman J et al. Inhibition of Rho via Arg and p190RhoGAP in the postnatal mouse hippocampus regulates dendritic spine maturation, synapse and dendrite stability, and behavior. J Neurosci 2007; 27: 10982–10992.
Basarsky TA, Parpura V, Haydon PG . Hippocampal synaptogenesis in cell culture: developmental time course of synapse formation, calcium influx, and synaptic protein distribution. J Neurosci 1994; 14 (11 Pt 1): 6402–6411.
Kalashnikova ELR, Kaur I, Barisone GA, Li B, Trimmer JS, Mohapatra DP et al. SynDIG1: an activity-regulated, AMPA- receptor-interacting transmembrane protein that regulates excitatory synapse development. Neuron 2010; 65: 13.
Wu Y, Wang W, Richerson GB . The transmembrane sodium gradient influences ambient GABA concentration by altering the equilibrium of GABA transporters. J Neurophysiol 2006; 96: 2425–2436.
Matus-Amat P, Higgins EA, Barrientos RM, Rudy JW . The role of the dorsal hippocampus in the acquisition and retrieval of context memory representations. J Neurosci 2004; 24: 2431–2439.
Radulovic J, Tronson NC . Molecular specificity of multiple hippocampal processes governing fear extinction. Rev Neurosci 2010; 21: 1–17.
Crawley JN . Designing mouse behavioral tasks relevant to autistic-like behaviors. Ment Retard Dev Disabil Res Rev 2004; 10: 248–258.
Nadler JJ, Moy SS, Dold G, Trang D, Simmons N, Perez A et al. Automated apparatus for quantitation of social approach behaviors in mice. Genes Brain Behav 2004; 3: 303–314.
Tabuchi K, Blundell J, Etherton MR, Hammer RE, Liu X, Powell CM et al. A neuroligin-3 mutation implicated in autism increases inhibitory synaptic transmission in mice. Science 2007; 318: 71–76.
Hung AY, Futai K, Sala C, Valtschanoff JG, Ryu J, Woodworth MA et al. Smaller dendritic spines, weaker synaptic transmission, but enhanced spatial learning in mice lacking Shank1. J Neurosci 2008; 28: 1697–1708.
Toro R, Konyukh M, Delorme R, Leblond C, Chaste P, Fauchereau F et al. Key role for gene dosage and synaptic homeostasis in autism spectrum disorders. Trends Genet 2010; 26: 363–372.
Graf ER, Zhang X, Jin SX, Linhoff MW, Craig AM . Neurexins induce differentiation of GABA and glutamate postsynaptic specializations via neuroligins. Cell 2004; 119: 1013–1026.
Rinaldi T, Silberberg G, Markram H . Hyperconnectivity of local neocortical microcircuitry induced by prenatal exposure to valproic acid. Cereb Cortex 2008; 18: 763–770.
Smith SE, Zhou YD, Zhang G, Jin Z, Stoppel DC, Anderson MP . Increased gene dosage of Ube3a results in autism traits and decreased glutamate synaptic transmission in mice. Sci Transl Med 2011; 3: 103ra97.
Nakatani J, Tamada K, Hatanaka F, Ise S, Ohta H, Inoue K et al. Abnormal behavior in a chromosome-engineered mouse model for human 15q11-13 duplication seen in autism. Cell 2009; 137: 1235–1246.
Neale BM, Kou Y, Liu L, Ma/'ayan A, Samocha KE, Sabo A et al. Patterns and rates of exonic de novo mutations in autism spectrum disorders. Nature 2012; 485: 242–245.
Sanders SJ, Murtha MT, Gupta AR, Murdoch JD, Raubeson MJ, Willsey AJ et al. De novo mutations revealed by whole-exome sequencing are strongly associated with autism. Nature 2012; 485: 237–241.
Hyman SE . A glimmer of light for neuropsychiatric disorders. Nature 2008; 455: 890–893.
Ben-Shachar S, Lanpher B, German JR, Qasaymeh M, Potocki L, Nagamani SC et al. Microdeletion 15q13.3: a locus with incomplete penetrance for autism, mental retardation, and psychiatric disorders. J Med Genet 2009; 46: 382–388.
Kim HG, Kishikawa S, Higgins AW, Seong IS, Donovan DJ, Shen Y et al. Disruption of neurexin 1 associated with autism spectrum disorder. Am J Hum Genet 2008; 82: 199–207.
Tennessen JA, Bigham AW, O'Connor TD, Fu W, Kenny EE, Gravel S et al. Evolution and functional impact of rare coding variation from deep sequencing of human exomes. Science 2012; 337: 64–69.
Lee S, Emond MJ, Bamshad MJ, Barnes KC, Rieder MJ, Nickerson DA et al. Optimal unified approach for rare-variant association testing with application to small-sample case-control whole-exome sequencing studies. Am J Hum Genet 2012; 91: 224–237.
Tuchman R, Cuccaro M . Epilepsy and autism: neurodevelopmental perspective. Curr Neurol Neurosci Rep 2011; 11: 428–434.
Etherton MR, Blaiss CA, Powell CM, Sudhof TC . Mouse neurexin-1alpha deletion causes correlated electrophysiological and behavioral changes consistent with cognitive impairments. Proc Natl Acad Sci USA 2009; 106: 17998–18003.
Missler M, Zhang W, Rohlmann A, Kattenstroth G, Hammer RE, Gottmann K et al. Alpha-neurexins couple Ca2+ channels to synaptic vesicle exocytosis. Nature 2003; 423: 939–948.
Acknowledgements
Thanks to Dr Margaret Price, Michael Lutter, Jeff Murray, Mathew State and Dr Vinu Mahajan for comments and insights regarding the paper. Thanks to Chantal Allamargot and Jean Ross in the University of Iowa Microscopy core for their technical assistance. This work was supported by NIH grant 1R01 NS064159-01A1 and a University of Iowa ICTS pilot-award (AGB).
Author contributions
LPS contributed to all experiments, planning and writing of the paper. LL performed all in vitro electrophysiology. YW performed in vivo electrophysiology. LL, LPS and DPM analyzed all the electrophysiology data. EC performed mouse behavioral experiments. YMU and JU performed a part of primary hippocampal neuron cultures. SW performed western blot experiments. JRM, HEL, LP, TW, XY and KM aided in the collection and sequencing of human DNA. PF, NU and SW conducted production and breeding of mice of different genotypes used in this study. AJS assisted in all immunocytochemistry experiments and analysis. GBR, DPM and JM provided reagents, supervised experiments and analysis, and aided in manuscript writing. JAW provided all behavioral equipment. JAW and DPM provided scientific input to the study design. BWD provided statistical analysis for whole-exome sequencing data. All authors contributed to the writing of this manuscript. AGB supervised all aspects of the project design, execution and writing of the paper. The data sets used for the analysis described in this manuscript were obtained from dbGaP at http://www.ncbi.nlm.nih.gov/gap through dbGaP accession number phs000298.v1.p1, under dbGAP Research Project #4357 (Variation in PRICKLE2 and related Genes in Autism) to AGB. The data set(s) were deposited by the ARRA Autism Sequencing Collaborative, an ARRA funded research initiative. Support for the Autism Sequencing Collaborative was provided by grants: R01-MH089208 awarded to Dr Mark Daly, R01-MH089175 awarded to Dr Richard Gibbs, R01-MH089025 awarded to Joseph Buxbaum, R01-MH089004 awarded to Gerard Schellenberg and R01-MH089482 awarded to James Sutcliffe. Embargo release date for the data was 29 February 2013.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Molecular Psychiatry website
Rights and permissions
About this article
Cite this article
Sowers, L., Loo, L., Wu, Y. et al. Disruption of the non-canonical Wnt gene PRICKLE2 leads to autism-like behaviors with evidence for hippocampal synaptic dysfunction. Mol Psychiatry 18, 1077–1089 (2013). https://doi.org/10.1038/mp.2013.71
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/mp.2013.71
Keywords
This article is cited by
-
Knockdown of the Non-canonical Wnt Gene Prickle2 Leads to Cerebellar Purkinje Cell Abnormalities While Cerebellar-Mediated Behaviors Remain Intact
The Cerebellum (2024)
-
The role of prickle proteins in vertebrate development and pathology
Molecular and Cellular Biochemistry (2023)
-
Celsr2 regulates NMDA receptors and dendritic homeostasis in dorsal CA1 to enable social memory
Molecular Psychiatry (2022)
-
PRICKLE2 revisited—further evidence implicating PRICKLE2 in neurodevelopmental disorders
European Journal of Human Genetics (2021)
-
Fyn depletion ameliorates tauP301L-induced neuropathology
Acta Neuropathologica Communications (2020)