Abstract
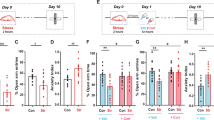
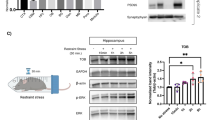
Hyperactivation of the amygdala following chronic stress is believed to be one of the primary mechanisms underlying the increased propensity for anxiety-like behaviors and pathological states; however, the mechanisms by which chronic stress modulates amygdalar function are not well characterized. The aim of the current study was to determine the extent to which the endocannabinoid (eCB) system, which is known to regulate emotional behavior and neuroplasticity, contributes to changes in amygdalar structure and function following chronic stress. To examine the hypothesis, we have exposed C57/Bl6 mice to chronic restraint stress, which results in an increase in fatty acid amide hydrolase (FAAH) activity and a reduction in the concentration of the eCB N-arachidonylethanolamine (AEA) within the amygdala. Chronic restraint stress also increased dendritic arborization, complexity and spine density of pyramidal neurons in the basolateral nucleus of the amygdala (BLA) and increased anxiety-like behavior in wild-type mice. All of the stress-induced changes in amygdalar structure and function were absent in mice deficient in FAAH. Further, the anti-anxiety effect of FAAH deletion was recapitulated in rats treated orally with a novel pharmacological inhibitor of FAAH, JNJ5003 (50 mg per kg per day), during exposure to chronic stress. These studies suggest that FAAH is required for chronic stress to induce hyperactivity and structural remodeling of the amygdala. Collectively, these studies indicate that FAAH-mediated decreases in AEA occur following chronic stress and that this loss of AEA signaling is functionally relevant to the effects of chronic stress. These data support the hypothesis that inhibition of FAAH has therapeutic potential in the treatment of anxiety disorders, possibly by maintaining normal amygdalar function in the face of chronic stress.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Phelps EA, LeDoux JE . Contributions of the amygdala to emotion processing: from animal models to human behavior. Neuron 2005; 48: 175–187.
van Marle HJ, Hermans EJ, Qin S, Fernandez G . From specificity to sensitivity: how acute stress affects amygdala processing of biologically salient stimuli. Biol Psychiatry 2009; 66: 649–655.
Pitkanen A, Savander V, LeDoux JE . Organization of intra-amygdaloid circuitries in the rat: an emerging framework for understanding functions of the amygdala. Trends Neurosci 1997; 20: 517–523.
McDonald AJ . Cortical pathways to the mammalian amygdala. Prog Neurobiol 1998; 55: 257–332.
Shekhar A, Truitt W, Rainnie D, Sajdyk T . Role of stress, corticotrophin releasing factor (CRF) and amygdala plasticity in chronic anxiety. Stress 2005; 8: 209–219.
Joels M, Baram TZ . The neuro-symphony of stress. Nat Rev Neurosci 2009; 10: 459–466.
Rainnie DG, Bergeron R, Sajdyk TJ, Patil M, Gehlert DR, Shekhar A . Corticotrophin releasing factor-induced synaptic plasticity in the amygdala translates stress into emotional disorders. J Neurosci 2004; 24: 3471–3479.
Rosenkranz JA, Venheim ER, Padival M . Chronic stress causes amygdala hyperexcitability in rodents. Biol Psychiatry 2010; 67: 1128–1136.
Sanders SK, Shekhar A . Anxiolytic effects of chlordiazepoxide blocked by injection of GABAA and benzodiazepine receptor antagonists in the region of the anterior basolateral amygdala of rats. Biol Psychiatry 1995; 37: 473–476.
Petersen EN, Braestrup C, Scheel-Kruger J . Evidence that the anticonflict effect of midazolam in amygdala is mediated by the specific benzodiazepine receptors. Neurosci Lett 1985; 53: 285–288.
Pesold C, Treit D . The central and basolateral amygdala differentially mediate the anxiolytic effects of benzodiazepines. Brain Res 1995; 671: 213–221.
Green S, Vale AL . Role of amygdaloid nuclei in the anxiolytic effects of benzodiazepines in rats. Behav Pharmacol 1992; 3: 261–264.
Sajdyk TJ, Shekhar A . Sodium lactate elicits anxiety in rats after repeated GABA receptor blockade in the basolateral amygdala. Eur J Pharmacol 2000; 394: 265–273.
Sanders SK, Shekhar A . Regulation of anxiety by GABAA receptors in the rat amygdala. Pharmacol Biochem Behav 1995; 52: 701–706.
Sajdyk TJ, Shekhar A . Excitatory amino acid receptors in the basolateral amygdala regulate anxiety responses in the social interaction test. Brain Res 1997; 764: 262–264.
Price JL, Drevets WC . Neurocircuitry of mood disorders. Neuropsychopharmacology 2010; 35: 192–216.
Shin LM, Liberzon I . The neurocircuitry of fear, stress, and anxiety disorders. Neuropsychopharmacology 2010; 35: 169–191.
McEwen BS . Physiology and neurobiology of stress and adaptation: central role of the brain. Physiol Rev 2007; 87: 873–904.
Buffalari DM, Grace AA . Chronic cold stress increases excitatory effects of norepinephrine on spontaneous and evoked activity of basolateral amygdala neurons. Int J Neuropsychopharmacol 2009; 12: 95–107.
Correll CM, Rosenkranz JA, Grace AA . Chronic cold stress alters prefrontal cortical modulation of amygdala neuronal activity in rats. Biol Psychiatry 2005; 58: 382–391.
Bennur S, Chattarji S . Effects of chronic stress on intrinsic and synaptic plasticity in principal neurons of the basolateral amygdala. Society for Neuroscience Abstracts 2004; 511.2.
Vyas A, Mitra R, Shankaranarayana Rao BS, Chattarji S . Chronic stress induces contrasting patterns of dendritic remodeling in hippocampal and amygdaloid neurons. J Neurosci 2002; 22: 6810–6818.
Vyas A, Pillai AG, Chattarji S . Recovery after chronic stress fails to reverse amygdaloid neuronal hypertrophy and enhanced anxiety-like behavior. Neuroscience 2004; 128: 667–673.
Vyas A, Jadhav S, Chattarji S . Prolonged behavioral stress enhances synaptic connectivity in the basolateral amygdala. Neuroscience 2006; 143: 387–393.
McEwen BS . Protection and damage from acute and chronic stress: allostasis and allostatic overload and relevance to the pathophysiology of psychiatric disorders. Ann NY Acad Sci 2004; 1032: 1–7.
Mitra R, Jadhav S, McEwen BS, Vyas A, Chattarji S . Stress duration modulates the spatiotemporal patterns of spine formation in the basolateral amygdala. Proc Natl Acad Sci USA 2005; 102: 9371–9376.
de Kloet ER, Joels M, Holsboer F . Stress and the brain: from adaptation to disease. Nat Rev Neurosci 2005; 6: 463–475.
Mitra R, Ferguson D, Sapolsky RM . SK2 potassium channel overexpression in basolateral amygdala reduces anxiety, stress-induced corticosterone secretion and dendritic arborization. Mol Psychiatry 2009; 14: 847–855, 827.
Mitra R, Sapolsky RM . Acute corticosterone treatment is sufficient to induce anxiety and amygdaloid dendritic hypertrophy. Proc Natl Acad Sci USA 2008; 105: 5573–5578.
Govindarajan A, Rao BS, Nair D, Trinh M, Mawjee N, Tonegawa S et al. Transgenic brain-derived neurotrophic factor expression causes both anxiogenic and antidepressant effects. Proc Natl Acad Sci USA 2006; 103: 13208–13213.
Freund TF, Katona I, Piomelli D . Role of endogenous cannabinoids in synaptic signaling. Physiol Rev 2003; 83: 1017–1066.
Ramikie TS, Patel S . Endocannabinoid signaling in the amygdala: anatomy, synaptic signaling, behavior, and adaptations to stress. Neuroscience 2012; 204: 38–52.
Patel S, Roelke CT, Rademacher DJ, Hillard CJ . Inhibition of restraint stress-induced neural and behavioural activation by endogenous cannabinoid signalling. Eur J Neurosci 2005; 21: 1057–1069.
Patel S, Kingsley PJ, Mackie K, Marnett LJ, Winder DG . Repeated homotypic stress elevates 2-arachidonoylglycerol levels and enhances short-term endocannabinoid signaling at inhibitory synapses in basolateral amygdala. Neuropsychopharmacology 2009; 34: 2699–2709.
Hill MN, McLaughlin RJ, Morrish AC, Viau V, Floresco SB, Hillard CJ et al. Suppression of amygdalar endocannabinoid signaling by stress contributes to activation of the hypothalamic-pituitary-adrenal axis. Neuropsychopharmacology 2009; 34: 2733–2745.
Hill MN, McLaughlin RJ, Bingham B, Shrestha L, Lee TT, Gray JM et al. Endogenous cannabinoid signaling is essential for stress adaptation. Proc Natl Acad Sci USA 2010; 107: 9406–9411.
Rademacher DJ, Meier SE, Shi L, Ho WS, Jarrahian A, Hillard CJ . Effects of acute and repeated restraint stress on endocannabinoid content in the amygdala, ventral striatum, and medial prefrontal cortex in mice. Neuropharmacology 2008; 54: 108–116.
Sumislawski JJ, Ramikie TS, Patel S . Reversible gating of endocannabinoid plasticity in the amygdala by chronic stress: a potential role for monoacylglycerol lipase inhibition in the prevention of stress-induced behavioral adaptation. Neuropsychopharmacology 2011; 36: 2750–2761.
Azad SC, Monory K, Marsicano G, Cravatt BF, Lutz B, Zieglgansberger W et al. Circuitry for associative plasticity in the amygdala involves endocannabinoid signaling. J Neurosci 2004; 24: 9953–9961.
Kodirov SA, Jasiewicz J, Amirmahani P, Psyrakis D, Bonni K, Wehrmeister M et al. Endogenous cannabinoids trigger the depolarization-induced suppression of excitation in the lateral amygdala. Learn Mem 2009; 17: 43–49.
Marsicano G, Wotjak CT, Azad SC, Bisogno T, Rammes G, Cascio MG et al. The endogenous cannabinoid system controls extinction of aversive memories. Nature 2002; 418: 530–534.
Shin RM, Tully K, Li Y, Cho JH, Higuchi M, Suhara T et al. Hierarchical order of coexisting pre- and postsynaptic forms of long-term potentiation at synapses in amygdala. Proc Natl Acad Sci USA 2010; 107: 19073–19078.
Chevaleyre V, Heifets BD, Kaeser PS, Sudhof TC, Castillo PE . Endocannabinoid-mediated long-term plasticity requires cAMP/PKA signaling and RIM1alpha. Neuron 2007; 54: 801–812.
Huang YC, Wang SJ, Chiou LC, Gean PW . Mediation of amphetamine-induced long-term depression of synaptic transmission by CB1 cannabinoid receptors in the rat amygdala. J Neurosci 2003; 23: 10311–10320.
Cravatt BF, Demarest K, Patricelli MP, Bracey MH, Giang DK, Martin BR et al. Supersensitivity to anandamide and enhanced endogenous cannabinoid signaling in mice lacking fatty acid amide hydrolase. Proc Natl Acad Sci USA 2001; 98: 9371–9376.
Omeir RL, Chin S, Hong Y, Ahern DG, Deutsch DG . Arachidonoyl ethanolamide-[1,2-14C] as a substrate for anandamide amidase. Life Sci 1995; 56: 1999–2005.
Hillard CJ, Wilkison DM, Edgemond WS, Campbell WB . Characterization of the kinetics and distribution of N-arachidonylethanolamine (anandamide) hydrolysis by rat brain. Biochim Biophys Acta 1995; 1257: 249–256.
Patel S, Carrier EJ, Ho WS, Rademacher DJ, Cunningham S, Reddy DS et al. The postmortal accumulation of brain N-arachidonylethanolamine (anandamide) is dependent upon fatty acid amide hydrolase activity. J Lipid Res 2005; 46: 342–349.
Johnson SA, Wang JF, Sun X, McEwen BS, Chattarji S, Young LT . Lithium treatment prevents stress-induced dendritic remodeling in the rodent amygdala. Neuroscience 2009; 163: 34–39.
Hill MN, Hillard CJ, McEwen BS . Alterations in corticolimbic dendritic morphology and emotional behavior in cannabinoid CB1 receptor-deficient mice parallel the effects of chronic stress. Cereb Cortex 2011; 21: 2056–2064.
Wellman CL, Izquierdo A, Garrett JE, Martin KP, Carroll J, Millstein R et al. Impaired stress-coping and fear extinction and abnormal corticolimbic morphology in serotonin transporter knock-out mice. J Neurosci 2007; 27: 684–691.
Feldman ML, Peters A . A technique for estimating total spine numbers on Golgi-impregnated dendrites. J Comp Neurol 1979; 188: 527–542.
Trommald M, Hulleberg G . Dimensions and density of dendritic spines from rat dentate granule cells based on reconstructions from serial electron micrographs. J Comp Neurol 1997; 377: 15–28.
Trommald M, Jensen V, Andersen P . Analysis of dendritic spines in rat CA1 pyramidal cells intracellularly filled with a fluorescent dye. J Comp Neurol 1995; 353: 260–274.
Horner CH, Arbuthnott E . Methods of estimation of spine density—are spines evenly distributed throughout the dendritic field? J Anat 1991; 177: 179–184.
Hill MN, Carrier EJ, McLaughlin RJ, Morrish AC, Meier SE, Hillard CJ et al. Regional alterations in the endocannabinoid system in an animal model of depression: effects of concurrent antidepressant treatment. J Neurochem 2008; 106: 2322–2336.
Mozhui K, Karlsson RM, Kash TL, Ihne J, Norcross M, Patel S et al. Strain differences in stress responsivity are associated with divergent amygdala gene expression and glutamate-mediated neuronal excitability. J Neurosci 2010; 30: 5357–5367.
Campolongo P, Roozendaal B, Trezza V, Cuomo V, Astarita G, Fu J et al. Fat-induced satiety factor oleoylethanolamide enhances memory consolidation. Proc Natl Acad Sci USA 2009; 106: 8027–8031.
Musella A, De Chiara V, Rossi S, Prosperetti C, Bernardi G, Maccarrone M et al. TRPV1 channels facilitate glutamate transmission in the striatum. Mol Cell Neurosci 2009; 40: 89–97.
Rossi S, De Chiara V, Musella A, Sacchetti L, Cantarella C, Castelli M et al. Preservation of striatal cannabinoid CB1 receptor function correlates with the antianxiety effects of fatty acid amide hydrolase inhibition. Mol Pharmacol 2010; 78: 260–268.
Gubellini P, Picconi B, Bari M, Battista N, Calabresi P, Centonze D et al. Experimental parkinsonism alters endocannabinoid degradation: implications for striatal glutamatergic transmission. J Neurosci 2002; 22: 6900–6907.
Gerdeman GL, Ronesi J, Lovinger DM . Postsynaptic endocannabinoid release is critical to long-term depression in the striatum. Nat Neurosci 2002; 5: 446–451.
Gulyas AI, Cravatt BF, Bracey MH, Dinh TP, Piomelli D, Boscia F et al. Segregation of two endocannabinoid-hydrolyzing enzymes into pre- and postsynaptic compartments in the rat hippocampus, cerebellum and amygdala. Eur J Neurosci 2004; 20: 441–458.
Domenici MR, Azad SC, Marsicano G, Schierloh A, Wotjak CT, Dodt HU et al. Cannabinoid receptor type 1 located on presynaptic terminals of principal neurons in the forebrain controls glutamatergic synaptic transmission. J Neurosci 2006; 26: 5794–5799.
Karst H, Berger S, Erdmann G, Schutz G, Joels M . Metaplasticity of amygdalar responses to the stress hormone corticosterone. Proc Natl Acad Sci USA 2010; 107: 14449–14454.
Haller J, Barna I, Barsvari B, Gyimesi Pelczer K, Yasar S, Panlilio LV et al. Interactions between environmental aversiveness and the anxiolytic effects of enhanced cannabinoid signaling by FAAH inhibition in rats. Psychopharmacology (Berl) 2009; 204: 607–616.
Naidu PS, Varvel SA, Ahn K, Cravatt BF, Martin BR, Lichtman AH . Evaluation of fatty acid amide hydrolase inhibition in murine models of emotionality. Psychopharmacology (Berl) 2007; 192: 61–70.
Patel S, Hillard CJ . Pharmacological evaluation of cannabinoid receptor ligands in a mouse model of anxiety: further evidence for an anxiolytic role for endogenous cannabinoid signaling. J Pharmacol Exp Ther 2006; 318: 304–311.
Moreira FA, Kaiser N, Monory K, Lutz B . Reduced anxiety-like behaviour induced by genetic and pharmacological inhibition of the endocannabinoid-degrading enzyme fatty acid amide hydrolase (FAAH) is mediated by CB1 receptors. Neuropharmacology 2008; 54: 141–150.
Hill MN, Karacabeyli ES, Gorzalka BB . Estrogen recruits the endocannabinoid system to modulate emotionality. Psychoneuroendocrinology 2007; 32: 350–357.
Bambico FR, Cassano T, Dominguez-Lopez S, Katz N, Walker CD, Piomelli D et al. Genetic deletion of fatty acid amide hydrolase alters emotional behavior and serotonergic transmission in the dorsal raphe, prefrontal cortex, and hippocampus. Neuropsychopharmacology 2010; 35: 2083–2100.
Hill MN, McEwen BS . Involvement of the endocannabinoid system in the neurobehavioural effects of stress and glucocorticoids. Prog Neuropsychopharmacol Biol Psychiatry 2010; 34: 791–797.
Di Marzo V, De Petrocellis L . Endocannabinoids as regulators of transient receptor potential (TRP) channels: a further opportunity to develop new endocannabinoid-based therapeutic drugs. Curr Med Chem 2010; 17: 1430–1449.
Hill MN, Miller GE, Ho WS, Gorzalka BB, Hillard CJ . Serum endocannabinoid content is altered in females with depressive disorders: a preliminary report. Pharmacopsychiatry 2008; 41: 48–53.
Sipe JC, Scott TM, Murray S, Harismendy O, Simon GM, Cravatt BF et al. Biomarkers of endocannabinoid system activation in severe obesity. PLoS One 2010; 5: e8792.
Hariri AR, Gorka A, Hyde LW, Kimak M, Halder I, Ducci F et al. Divergent effects of genetic variation in endocannabinoid signaling on human threat- and reward-related brain function. Biol Psychiatry 2009; 66: 9–16.
Hill MN, Hillard CJ, Bambico FR, Patel S, Gorzalka BB, Gobbi G . The therapeutic potential of the endocannabinoid system for the development of a novel class of antidepressants. Trends Pharmacol Sci 2009; 30: 484–493.
Gaetani S, Dipasquale P, Romano A, Righetti L, Cassano T, Piomelli D et al. The endocannabinoid system as a target for novel anxiolytic and antidepressant drugs. Int Rev Neurobiol 2009; 85: 57–72.
Acknowledgements
We thank Katharine McCarthy, Ilia Karatsoreos and Melinda Miller for their technical assistance on the execution of these studies. This research was supported by operating grants from the National Institutes of Health grants MH41256 (BSM), DA09155 and DA026996 (CJH) and the Lightfighter Trust (BSM), as well as an unrestricted research grant from Johnson and Johnson (BSM). SC and SAK are supported by funds from the National Centre for Biological Sciences (NCBS). MNH was the recipient of a postdoctoral fellowship from the Canadian Institutes of Health Research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
This research was supported, in part, by an unrestricted operating grant to BSM from Johnson and Johnson Pharmaceuticals. JMK is an employee of Janssen Research and Development LLC, of Johnson and Johnson Pharmaceuticals. The funding body had no role in the design of this study, collection or analysis of the data or decision to publish, outside of the development of the pharmacological compound, JNJ-5003, which was developed and used in a portion of this study.
Additional information
Supplementary Information accompanies the paper on the Molecular Psychiatry website
Supplementary information
Rights and permissions
About this article
Cite this article
Hill, M., Kumar, S., Filipski, S. et al. Disruption of fatty acid amide hydrolase activity prevents the effects of chronic stress on anxiety and amygdalar microstructure. Mol Psychiatry 18, 1125–1135 (2013). https://doi.org/10.1038/mp.2012.90
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/mp.2012.90
Keywords
This article is cited by
-
Inhibition of fatty acid binding protein-5 in the basolateral amygdala induces anxiolytic effects and accelerates fear memory extinction
Psychopharmacology (2024)
-
NAPE-PLD deletion in stress-TRAPed neurons results in an anxiogenic phenotype
Translational Psychiatry (2023)
-
Endocannabinoids at the synapse and beyond: implications for neuropsychiatric disease pathophysiology and treatment
Neuropsychopharmacology (2023)
-
Fatty acid amide hydrolase inhibition alleviates anxiety-like symptoms in a rat model used to study post-traumatic stress disorder
Psychopharmacology (2023)
-
Acute gut inflammation reduces neural activity and spine maturity in hippocampus but not basolateral amygdala
Scientific Reports (2022)