Abstract
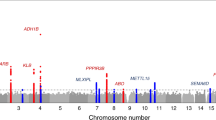
Dietary preference for fat may increase risk for obesity. It is a complex behavior regulated in part by the amygdala, a brain structure involved in reward processing and food behavior, and modulated by genetic factors. Here, we conducted a genome-wide association study (GWAS) to search for gene loci associated with dietary intake of fat, and we tested whether these loci are also associated with adiposity and amygdala volume. We studied 598 adolescents (12–18 years) recruited from the French–Canadian founder population and genotyped them with 530 011 single-nucleotide polymorphisms. Fat intake was assessed with a 24-hour food recall. Adiposity was examined with anthropometry and bioimpedance. Amygdala volume was measured by magnetic resonance imaging. GWAS identified a locus of fat intake in the μ-opioid receptor gene (OPRM1, rs2281617, P=5.2 × 10−6), which encodes a receptor expressed in the brain-reward system and shown previously to modulate fat preference in animals. The minor OPRM1 allele appeared to have a ‘protective’ effect: it was associated with lower fat intake (by 4%) and lower body-fat mass (by ∼2 kg, P=0.02). Consistent with the possible amygdala-mediated inhibition of fat preference, this allele was additionally associated with higher amygdala volume (by 69 mm3, P=0.02) and, in the carriers of this allele, amygdala volume correlated inversely with fat intake (P=0.02). Finally, OPRM1 was associated with fat intake in an independent sample of 490 young adults. In summary, OPRM1 may modulate dietary intake of fat and hence risk for obesity, and this effect may be modulated by subtle variations in the amygdala volume.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Klesges RC, Klesges LM, Haddock CK, Eck LH . A longitudinal analysis of the impact of dietary intake and physical activity on weight change in adults. Am J Clin Nutr 1992; 55: 818–822.
Tucker LA, Seljaas GT, Hager RL . Body fat percentage of children varies according to their diet composition. J Am Diet Assoc 1997; 97: 981–986.
Bray GA, Popkin BM . Dietary fat intake does affect obesity!. Am J Clin Nutr 1998; 68: 1157–1173.
Herberg L, Doppen W, Major E, Gries FA . Dietary-induced hypertrophic–hyperplastic obesity in mice. J Lipid Res 1974; 15: 580–585.
Jen KL . Effects of diet composition on food intake and carcass composition in rats. Physiol Behav 1988; 42: 551–556.
Levin BE, Triscari J, Sullivan AC . Metabolic features of diet-induced obesity without hyperphagia in young rats. Am J Physiol 1986; 251 (3 Part 2): R433–R440.
Oscai LB, Brown MM, Miller WC . Effect of dietary fat on food intake, growth and body composition in rats. Growth 1984; 48: 415–424.
Oscai LB, Miller WC, Arnall DA . Effects of dietary sugar and of dietary fat on food intake and body fat content in rats. Growth 1987; 51: 64–73.
Hill JO, Peters JC . Environmental contributions to the obesity epidemic. Science 1998; 280: 1371–1374.
Rubner M . Der energiewert der Kost des Menschen. Z Biol 1901; 42: 261.
Tappy L . Thermic effect of food and sympathetic nervous system activity in humans. Reprod Nutr Dev 1996; 36: 391–397.
Kenny PJ . Reward mechanisms in obesity: new insights and future directions. Neuron 2011; 69: 664–679.
Kenny PJ . Common cellular and molecular mechanisms in obesity and drug addiction. Nat Rev Neurosci 2011; 12: 638–651.
Hill SY . Trajectories of alcohol use and electrophysiological and morphological indices of brain development: distinguishing causes from consequences. Ann N Y Acad Sci 2004; 1021: 245–259.
Wrase J, Makris N, Braus DF, Mann K, Smolka MN, Kennedy DN et al. Amygdala volume associated with alcohol abuse relapse and craving. Am J Psychiatry 2008; 165: 1179–1184.
Hill SY, De Bellis MD, Keshavan MS, Lowers L, Shen S, Hall J et al. Right amygdala volume in adolescent and young adult offspring from families at high risk for developing alcoholism. Biol Psychiatry 2001; 49: 894–905.
Primeaux SD, York DA, Bray GA . Neuropeptide Y administration into the amygdala alters high fat food intake. Peptides 2006; 27: 1644–1651.
Boghossian S, Park M, York DA . Melanocortin activity in the amygdala controls appetite for dietary fat. Am J Physiol Regul Integr Comp Physiol 2010; 298: R385–R393.
Grabenhorst F, Rolls ET, Parris BA, d'Souza AA . How the brain represents the reward value of fat in the mouth. Cereb Cortex 2010; 20: 1082–1091.
Haghighi A, Schwartz DH, Abrahamowicz M, Leonard GT, Perron M, Richer L et al. Prenatal exposure to maternal cigarette smoking, amygdala volume and fat intake in adolescence. Arch Gen Psychiatry 2012; 3: 1–8.
Reed DR, Bachmanov AA, Beauchamp GK, Tordoff MG, Price RA . Heritable variation in food preferences and their contribution to obesity. Behav Genet 1997; 27: 373–387.
Cai G, Cole SA, Bastarrachea RA, Maccluer JW, Blangero J, Comuzzie AG . Quantitative trait locus determining dietary macronutrient intakes is located on human chromosome 2p22. Am J Clin Nutr 2004; 80: 1410–1414.
Choquette AC, Lemieux S, Tremblay A, Chagnon YC, Bouchard C, Vohl MC et al. Evidence of a quantitative trait locus for energy and macronutrient intakes on chromosome 3q27.3: the Quebec Family Study. Am J Clin Nutr 2008; 88: 1142–1148.
Collaku A, Rankinen T, Rice T, Leon AS, Rao DC, Skinner JS et al. A genome-wide linkage scan for dietary energy and nutrient intakes: the Health, Risk Factors, Exercise Training, and Genetics (HERITAGE) Family Study. Am J Clin Nutr 2004; 79: 881–886.
Smith Richards BK, Belton BN, Poole AC, Mancuso JJ, Churchill GA, Li R et al. QTL analysis of self-selected macronutrient diet intake: fat, carbohydrate, and total kilocalories. Physiol Genomics 2002; 11: 205–217.
Bauer F, Elbers CC, Adan RA, Loos RJ, Onland-Moret NC, Grobbee DE et al. Obesity genes identified in genome-wide association studies are associated with adiposity measures and potentially with nutrient-specific food preference. Am J Clin Nutr 2009; 90: 951–959.
De Braekeleer M . Hereditary disorders in Saguenay-Lac-St-Jean (Quebec, Canada). Hum Hered 1991; 41: 141–146.
Grompe M, St-Louis M, Demers SI, al-Dhalimy M, Leclerc B, Tanguay RM . A single mutation of the fumarylacetoacetate hydrolase gene in French Canadians with hereditary tyrosinemia type I. N Engl J Med 1994; 331: 353–357.
De Braekeleer M, Mari C, Verlingue C, Allard C, Leblanc JP, Simard F et al. Complete identification of cystic fibrosis transmembrane conductance regulator mutations in the CF population of Saguenay Lac-Saint-Jean (Quebec, Canada). Clin Genet 1998; 53: 44–46.
Peltonen L, Palotie A, Lange K . Use of population isolates for mapping complex traits. Nat Rev Genet 2000; 1: 182–190.
Pausova Z, Paus T, Abrahamowicz M, Almerigi J, Arbour N, Bernard M et al. Genes, maternal smoking, and the offspring brain and body during adolescence: design of the Saguenay Youth Study. Hum Brain Mapp 2007; 28: 502–518.
Thompson FE, Subar AF . Dietary assessment methodology. In: Bousky C, (ed). Nutrition in the Prevention and Treatment of Disease 2nd edn Academic Press, 2008.
Buzzard IM, Faucett CL, Jeffery RW, McBane L, McGovern P, Baxter JS et al. Monitoring dietary change in a low-fat diet intervention study: advantages of using 24-hour dietary recalls vs food records. J Am Diet Assoc 1996; 96: 574–579.
Berthiaume P, Lavalee C, Villeneuve M, Vigneaut M . Enquête sociale et de santé auprès des enfants et des adolescents québécois, In nutrition V, (ed). Les Publications du Québec, 2004 pp 19–33.
Fischl B, Dale AM . Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci USA 2000; 97: 11050–11055.
Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C et al. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron 2002; 33: 341–355.
Price AL, Patterson NJ, Plenge RM, Weinblatt ME, Shadick NA, Reich D . Principal components analysis corrects for stratification in genome-wide association studies. Nat Genet 2006; 38: 904–909.
Abecasis GR, Cherny SS, Cookson WO, Cardon LR . Merlin–rapid analysis of dense genetic maps using sparse gene flow trees. Nat Genet 2002; 30: 97–101.
Abecasis GR, Wigginton JE . Handling marker-marker linkage disequilibrium: pedigree analysis with clustered markers. Am J Hum Genet 2005; 77: 754–767.
Lander ES, Green P . Construction of multilocus genetic linkage maps in humans. Proc Natl Acad Sci USA 1987; 84: 2363–2367.
Cahill LE, El-Sohemy A . Haptoglobin genotype modifies the association between dietary vitamin C and serum ascorbic acid deficiency. Am J Clin Nutr 2010; 92: 1494–1500.
Yu A, Zhao C, Fan Y, Jang W, Mungall AJ, Deloukas P et al. Comparison of human genetic and sequence-based physical maps. Nature 2001; 409: 951–953.
Chanock SJ, Manolio T, Boehnke M, Boerwinkle E, Hunter DJ, Thomas G et al. Replicating genotype-phenotype associations. Nature 2007; 447: 655–660.
Pecina S, Smith KS . Hedonic and motivational roles of opioids in food reward: implications for overeating disorders. Pharmacol Biochem Behav 2010; 97: 34–46.
Gosnell BA, Levine AS . Reward systems and food intake: role of opioids. Int J Obes (Lond) 2009; 33 (Suppl 2): S54–S58.
Zhang M, Gosnell BA, Kelley AE . Intake of high-fat food is selectively enhanced by mu opioid receptor stimulation within the nucleus accumbens. J Pharmacol Exp Ther 1998; 285: 908–914.
Barnes MJ, Argyropoulos G, Bray GA . Preference for a high fat diet, but not hyperphagia following activation of mu opioid receptors is blocked in AgRP knockout mice. Brain Res 2010; 1317: 100–107.
Zheng H, Townsend RL, Shin AC, Patterson LM, Phifer CB, Berthoud HR . High-fat intake induced by mu-opioid activation of the nucleus accumbens is inhibited by Y1R-blockade and MC3/4R- stimulation. Brain Res 2010; 1350: 131–138.
Taha SA . Preference or fat? Revisiting opioid effects on food intake. Physiol Behav 2010; 100: 429–437.
Dlugos AM, Hamidovic A, Hodgkinson C, Shen PH, Goldman D, Palmer AA et al. OPRM1 gene variants modulate amphetamine-induced euphoria in humans. Genes Brain Behav 2011; 10: 199–209.
Zhu W, Pan ZZ . Mu-opioid-mediated inhibition of glutamate synaptic transmission in rat central amygdala neurons. Neuroscience 2005; 133: 97–103.
Tong Y, Chabot JG, Shen SH, O'Dowd BF, George SR, Quirion R . Ontogenic profile of the expression of the mu opioid receptor gene in the rat telencephalon and diencephalon: an in situ hybridization study. J Chem Neuroanat 2000; 18: 209–222.
Khurshid N, Hameed LS, Mohanasundaram S, Iyengar S . Opioid modulation of cell proliferation in the ventricular zone of adult zebra finches (Taenopygia guttata). FASEB J 2010; 24: 3681–3695.
Neel JV . Diabetes mellitus: a ‘thrifty’ genotype rendered detrimental by ‘progress’? Am J Hum Genet 1962; 14: 353–362.
Eaton SB, Konner M . Paleolithic nutrition. A consideration of its nature and current implications. N Engl J Med 1985; 312: 283–289.
Acknowledgements
We thank the following individuals for their contributions in acquiring data: Jacynthe Tremblay and her team of research nurses (Saguenay Hospital), Helene Simard and her team of research assistants (Cégep de Jonquière) and Dr Rosanne Aleong (program manager, Rotman Research Institute). The Canadian Institutes of Health Research (ZP, TP) funds the SYS. MA is a James McGill Professor of Biostatistics at McGill University. TP is the Tanenbaum Chair in Population Neuroscience at the Rotman Research Institute, University of Toronto.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Molecular Psychiatry website
Supplementary information
Rights and permissions
About this article
Cite this article
Haghighi, A., Melka, M., Bernard, M. et al. Opioid receptor mu 1 gene, fat intake and obesity in adolescence. Mol Psychiatry 19, 63–68 (2014). https://doi.org/10.1038/mp.2012.179
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/mp.2012.179
Keywords
This article is cited by
-
Abnormalities in deep-brain morphology and orbitofrontal cortical thinning relate to reward processing and body mass in adolescent girls
International Journal of Obesity (2022)
-
Hyperpalatability and the Generation of Obesity: Roles of Environment, Stress Exposure and Individual Difference
Current Obesity Reports (2018)
-
Interactions of early adversity with stress-related gene polymorphisms impact regional brain structure in females
Brain Structure and Function (2016)
-
Involvement of Endogenous Enkephalins and β-Endorphin in Feeding and Diet-Induced Obesity
Neuropsychopharmacology (2015)
-
Genetic Similarities between Compulsive Overeating and Addiction Phenotypes: A Case for “Food Addiction”?
Current Psychiatry Reports (2015)