Abstract
The activation of glucocorticoid receptors (GR) by glucocorticoids increases stress-related memory through the activation of the MAPK signaling pathway and the downstream transcription factor Egr-1. Here, using converging in vitro and in vivo approaches, respectively, GR-expressing cell lines, culture of hippocampal neurons, and GR genetically modified mice (GRNesCre), we identified synapsin-Ia/Ib as one of the effectors of the glucocorticoid signaling cascade. Stress and glucocorticoid-induced activation of the GR modulate synapsin-Ia/Ib through two complementary mechanisms. First, glucocorticoids driving Egr-1 expression increase the expression of synapsin-Ia/Ib, and second, glucocorticoids driving MAPK activation increase its phosphorylation. Finally, we showed that blocking fucosylation of synapsin-Ia/Ib in the hippocampus inhibits its expression and prevents the glucocorticoid-mediated increase in stress-related memory. In conclusion, our data provide a complete molecular pathway (GR/Egr-1/MAPK/Syn-Ia/Ib) through which stress and glucocorticoids enhance the memory of stress-related events and highlight the function of synapsin-Ia/Ib as molecular effector of the behavioral effects of stress.
Similar content being viewed by others
Introduction
Glucocorticoid hormones are released by the adrenal glands during the active phase of the circadian cycle and in response to stress.1, 2, 3, 4 Stress-induced high levels of glucocorticoids have been shown to increase the memory of stress-associated events5, 6, 7, 8 and to contribute to the development of stress-related pathologies such as depression, anxiety, and drug abuse.1, 3, 4, 5, 6, 9, 10 Most of these behavioral effects of stress-induced glucocorticoid secretion depend on the activation of glucocorticoid receptors (GR).11 GR are steroid-regulated transcription factors, which on binding to glucocorticoids are translocated to the nucleus in which they modify transcription by multiple mechanisms.12 Consequently, identifying the transcriptional targets of stress-induced activation of GR is paramount in uncovering the molecular basis of stress-related disorders.
In an earlier paper, we described how activated GR enhance fear memories by inducing a cascade of events that starts with increasing the expression of the transcription factor Egr-1 followed by an increase in expression and activity of the MAPK pathway, which further enhances the expression of Egr1.8 These effects were observed in the hippocampus, one of the main brain targets of glucocorticoids that is crucial for the encoding of memory.5, 6 The GR/Egr-1/MAPK was the first molecular cascade subserving the enhancement of stress-related memory to be identified.8 Unfortunately, this cascade starts with a transcriptional factor, GR, and ends up with another transcription factor, Egr-1.8 Consequently, the ultimate effector proteins through which stress induces an enhancement of memory still remain unknown.
In this report, we show that, in the hippocampus, synapsin-Ia/Ib isoforms are one of the effector proteins through which the GR/Egr-1/MAPK cascade enhances stress-related memories. Synapsin-Ia/Ib are the most highly expressed hippocampal pre-synaptic vesicle (SV)-associated phosphoproteins.13, 14 The mammalian synapsin family is composed of three genes (syn1, syn2, and syn3) that are alternatively spliced to give rise to two isoforms a and b regulated by phosphorylation.13 Synapsins control the release of neurotransmitters, such as glutamate, which has a crucial function in synaptic plasticity.15, 16, 17 These proteins have also been involved in the memory process in drosophila and in aging-related memory impairment in mammals.18, 19, 20 However, the function of the different synapsin isoforms in the formation of specific types of memories remains unknown.
The expression of synapsin-Ia/Ib and their phosphorylation counterparts in response to glucocorticoids was studied using PC12 cell lines that express both endogenous GR and synapsin isoforms,21, 22 primary culture of hippocampal neurons, as well as the hippocampus of GR genetically modified mice (GRNesCre) in which the expression of GR has been conditionally suppressed.8, 12, 23 The function of synapsin-Ia/Ib on stress-related memory was investigated using 2-deoxy-D-galactose (2-dGal), an inhibitor of synapsin-Ia/Ib expression.24
Our results show that the GR/Egr-1/MAPK signaling cascade increases stress-related memories through synapsin-Ia/Ib. GR-activated Egr-1 increases the expression of synapsin-Ia/Ib, the function of which is to enhance the pool of readily releasable SVs. GR-activated MAPK then increases synapsin phosphorylation, the function of which is to induce neurotransmitter release. Finally, inhibition of GR-induced increase in hippocampal synapsin-I expression abolishes the enhancement of stress-related memories mediated by the GR/Egr-1/MAPK signaling pathway. Together, our studies identify a complete molecular pathway (GR/Egr-1/MAPK/Syn-Ia/Ib, GEMS) enhancing the consolidation of stress-related memory and highlight the important function of synapsin-Ia/Ib as an effector of the behavioral effects of glucocorticoids.
Materials and methods
Study of the interaction between GR and synapsin in vivo
For all the experiments, 5–6-week-old mice were fed ad libitum and maintained in a temperature (22±1 °C) and humidity (60±5%)-controlled environment under a 12-h light/dark cycle (lights on at 0800 hours). The gr gene (also called Nr3c1) was knocked out using the Cre-LoxP recombination system in which the Cre recombinase under the control of the rat nestin promoter and enhancer allows GRLoxP allele excision in the entire brain of mutant mice,23 but not in other tissues. Control littermates not expressing Cre were GRLoxP/LoxP mice. Experiments carried out in basal conditions compared GRLoxP/LoxP and their littermates, GRNesCre mice. The mice were killed when glucocorticoid levels were low (beginning of the inactive phase of the light cycle (0900 hours)). For the stress experiments, GRLoxP/LoxP (n=4) and GRNesCre (n=4) mice were subjected to a 30-min restraint stress and killed either in basal conditions (t0) or 30 and 120 min after stress onset. Then, the hippocampus and blood were collected, respectively, and assayed either for protein extraction or for corticosterone assay. All the experiments were conducted in strict compliance with the European Communities Council Directive of 24 November 1986 (86/609/EEC) and approved by the Aquitaine-Poitou Charentes ethical committee.
Chemicals
The corticosterone used in the various experiments was from Sigma (C-2505, St Louis, MO, USA). Corticosterone was used at 10 nM in PC12 cells and at 100 nM in primary culture of hippocampal neurons. The latter concentration was chosen on the basis of preliminary experiments showing that 100 nM corresponded to the EC100 for the nuclear translocation of the GR in this cellular model. MEK1/2 inhibitor (UO126, 9903, Cell Signaling Technology, Danvers, MA, USA) at a final concentration of 10 μM was used to block the MAPK signaling pathway. 2-dGal (259580, Calbiochem, Gibbstown, NJ, USA), acting as an inhibitor of synapsin fucosylation, was used at a final concentration of 400 mM per hemisphere.
Cell culture and transient siRNA transfection
The PC12 cell line (ATCC CRL-1721, Manassas, VA, USA) derived from a transplantable rat pheochromocytoma was used. The PC12 cells were trypsinized and seeded on poly-D-lysine-coated wells to the appropriate concentration (9 × 105 cells per well) in fresh, antibiotic-free medium (15% horse serum+2.5% fetal bovine serum). Transfections of siRNA ON-TARGETplus SMARTpool against Egr-1 (10 nmol, L-100247-01-0010, Dharmacon, Lafayette, CO, USA), siRNA-negative control ON-TARGETplus siCONTROL non-targeting pool (10 nmol, D-001810-10-05, Dharmacon), and siRNA-Alexa Fluor 546 (1027098, Qiagen, Duesseldorf, Germany) were performed with Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) within OPTI-MEM medium (Invitrogen) according to the manufacturer's instructions at a final ratio of 1:1. The percentage of transfection efficiency realized with siRNA-Alexa Fluor 546 was determined by flow cytometry (FACS Calibur, BD, USA). Then 6 h after transfection, the OPTI-MEM medium was changed for a steroid-free culture medium. After 65 h post-transfection, the cells were treated for 1 h with 10 nM of corticosterone-hydroxypropyl-β-cyclodextrin (Sigma); proteins were extracted with RIPA buffer containing protease and phosphatase inhibitors and subjected to western blot analysis.
Cultures of hippocampal neurons prepared from E18 Sprague–Dawley rats were plated at a density of 200 × 103 cells per ml on poly-lysine pre-coated coverslips.25 Cultures were maintained in serum-free N2 medium26 and kept at 36.5 °C in 5.0% CO2 for 7–9 days in vitro before transfection. Neuronal Egr-1 down-regulation by RNA interference was performed by co-transfecting EGFP cDNA (1.6 μg) as a reporter and RNA duplexes (2.4 μg) with Lipofectamine 2000 (15 μl, Invitrogen) according to the manufacturer's instructions. Neurons were incubated for 1 h with this solution in serum-free N2 medium. Forty eight hours after transfection, the neurons were treated for 1 h with 100 nM of corticosterone-hydroxypropyl-β-cyclodextrin (Sigma) and then siRNA efficiency was checked by immunostaining.
Immunostaining
Neurons were fixed with 4% paraformaldehyde (10 min room temperature), washed, and permeabilized using 0.1% Triton-X 100 (5 min room temperature; Sigma). The cells were incubated with primary antibodies for 1 h and then with secondary antibodies for 30 min at room temperature. After washing, neurons were mounted with Mowiol (Calbiochem) or ProLong Gold Antifade Reagent containing DAPI (Molecular Probes–Invitrogen, Eugene, OR, USA). Preparations were kept at 4 °C until analysis. GR immunoreactivity, Egr-1-immunoreactivity, and synapsin-I-immunoreactivity were revealed using a rabbit polyclonal anti-GR antibody (sc-1004-X, 1/500, Santa Cruz, Santa Cruz, CA, USA), a rabbit polyclonal anti-Egr-1 antibody (ARP32241_P50, 1/500, Aviva System biology, CA, USA) and a mouse monoclonal anti-synapsin-I (106001, 1/400, SYSY: Synaptic System, Germany), respectively. Secondary antibodies with fluorescent labels were from Molecular Probes (UK). Microscopic imaging was performed using an upright epi-fluorescence microscope Leica DMR (Leica Microsystems, Wetzlar, Germany) equipped with objective HCX PL APO 63X oil NA 1.32 and a CoolSnapHQ camera (Roper Scientific, Evry, France). Quantification of the fluorescence was realized using imaging tools from Metamorph software (Universal Imaging Corporation, PA, USA).
Protein extraction from cell culture and tissues
Nuclear and cytoplasmic protein extracts from PC12 cell lines and from mice hippocampi containing protease and phosphatase inhibitors (Sigma) were prepared either using a procedure earlier described and validated27 or using the homogenizer Precellys 24, which is a benchtop device dedicated to the grinding, lysis, and homogenization of biological samples (Bertin Technologies, Montigny-le-Bretonneux, France). The homogenizing protocol in RIPA buffer containing protease and phosphatase inhibitors was 5000 r.p.m. 2 × 30+10 s break using ceramic CK14 beads (Bertin Technologies).
Immunoblotting analysis
Cytoplasmic and nuclear proteins suspended in Laemmli buffer were separated by SDS–PAGE (10% gels) and transferred onto PVDF membranes (Immobilon P, IPVH00010, Millipore, Billerica, MA, USA). The proteins were revealed with relevant antibodies as described earlier.27 The following Santa Cruz polyclonal antibodies were used: anti-GR (sc-1004-X; 1/50 000 dilution), anti-Egr-1 (sc-189; 1/1000 dilution), anti-Synapsin-Ia/Ib (sc-8295; 1/2000 dilution), anti-Synapsin-IIa (sc-8293; 1/2000 dilution), and anti-Synapsin-IIIa (sc-8292; 1/350 dilution). Aviva System biology supplied the rabbit polyclonal anti-Egr-1 (ARP36841_T100; 1/4000 dilution). BD Biosciences (Franklin Lakes, NJ, USA) supplied the monoclonal anti-Synapsin-I (611392; 1/10 000 dilution) and anti-Synapsin-IIa (610666; 1/2000 dilution). Upstate (Charlottesville, VA, USA) supplied the polyclonal anti-MAPK (06-182; 1/100 000 dilution). Polyclonal anti-Phospho-MAPK (9101S; 1/1000 dilution) and Phospho-Synapsin (2311; 1/1000 dilution), and Pan-synapsin (2312; 1/2000 dilution) were from Cell Signaling Technology. The Neuronal Class III β-tubulin (TUJ1) monoclonal antibody (MMS-435P; 1/20 000 dilution) was purchased from Eurogentec (Seraing, Belgium). The X-ray films (Kodak, USA) were quantified by densitometry using a GS-800 scanner (in transmission mode) and the associated Quantity One software (Bio-Rad, CA, USA) according to the manufacturer's instructions.
Corticosterone assay
Plasma corticosterone was quantified by radioimmunoassay using a specific corticosterone antibody (ICN Pharmaceuticals, CA, USA) as described elsewhere.27
Behavioral experiments
Subjects and surgery
C57/Bl6 male mice (n=69, 3–4 months old (IFFA Credo, Arbresle, France) were anesthetized with ketamine/xylazine (Bayer, Leverkusen, Germany) (8 ml kg–1, intra-peritoneally). Using a Kopf stereotaxic apparatus, stainless-steel guide cannulae (26-gauge, 8 mm length) were implanted bilaterally 1 mm above the dorsal hippocampus and fixed in place with dental cement. Mice were allowed to recover for at least 8 days before the behavioral experiments.
Contextual fear conditioning procedure
All experimental procedures took place during the light portion of the light/dark cycle. Mice were handled daily for 5 min 3 days before the start of the contextual conditioning procedure. This behavioral procedure has been repeatedly used and fully described in earlier studies.8, 28 Briefly, each animal was placed in the conditioning chamber for 4 min during which it received two footshocks, which never co-occurred with two tone (63 db, 1 kHz, 15 s) delivery. Each animal was then returned to its home cage. Twenty-four hours later, the mice were re-exposed to the conditioning chamber and the behavior of the subjects was continuously recorded on videotape for off-line scoring of freezing. The conditioning procedure consisted of a tone (CS)-shock (US) unpairing known to favor a context-US association.28 Animals received one of two shock regimens: either 0.3 mA, 50 Hz, 3 s (low shock intensity group) or 0.7 mA, 50 Hz, 3 s (high shock intensity group). Freezing behavior, used as an index of conditioned fear, was calculated as a percentage (±s.e.m.) of the total time spent freezing during the first 4 min-period of the retention test.
Microinjections
Immediately after the acquisition of fear conditioning, animals were placed in their home cage and randomly divided into four groups according to the intra-hippocampal infusion they received: (1) vehicle (1% DMSO in artificial cerebrospinal fluid), (2) corticosterone (10 ng per side), (3) 2-dGal (400 mM per side), and (4) corticosterone+2-dGal. Infusions (0.3 μl per hemisphere) were performed at a constant rate (0.1 μl min–1) through 32-gauge stainless-steel cannulae (9 mm) that were inserted into the guide cannulae and left in place for 2 min after the end of the infusion. Corticosterone (2-hydroxypropyl-β-cyclodextrin complex; Sigma-Aldrich, St Louis, MO, USA) and 2-dGal were dissolved in artificial cerebrospinal fluid (145 mM NaCl, 2.7 mM KCl, 1 mM MgCl2, 1.2 mM CaCl2, and 0.2 mM Na2HPO4/NaH2PO4 buffered at pH 7.4).
Histology
After completion of the behavioral study, animals were given an overdose of ketamin (24 ml kg–1) and transcardially perfused with physiological saline, followed by 10% buffered formalin. Brains were post-fixed in formalin-saccharose 30% solution for 1 week, frozen, cut coronally on a sliding microtome into 50 μm sections that were mounted on a gelatin-coated slide and stained with thionine to evaluate the cannulae placements.
Statistics
Statistical analyses were performed using analysis of variance (ANOVA) followed by the Newman–Keuls, Duncan, or Scheffe post hoc test for pairwise comparisons when appropriate. The Student's t-test was used for pairwise comparisons.
Results
GR in the hippocampus regulate synapsin-I expression and phosphorylation
Using western blot, we compared the expression of members of the synapsin family in the hippocampus of GRNesCre mice, in which the expression of the GR protein is suppressed in the entire brain, but not in other tissues using the Cre/LoxP system23 and in control littermates.
In basal conditions, using pan-synapsin, an antibody that detects all synapsin isoforms, we found a reduced expression of these proteins and of their phosphorylated counterparts in GRNesCre mutant mice. However, when more specific antibodies were used, we found a selective decrease in the expression of synapsin-Ia/Ib, but not of synapsin-IIa and -IIIa (Figure 1a and b). Finally, levels of the transcription factor Egr-1 were also reduced in GRNesCre mutant mice.8
Comparison of the expression of the synapsin proteins family and Egr-1 under basal condition and after stress between wild-type (WT) and GRNesCre mice in which the glucocorticoid receptors (GR) were suppressed in the entire brain. (a) Western blot analyses of hippocampus extracts for GR, Egr-1, phosphorylated-synapsin (P-synapsin), total synapsin (Pan-synapsin), synapsin-Ia/Ib, synapsin-IIa, and synapsin-IIIa isoforms were performed using specific antibodies. βIII-tubulin was used as a loading control. (b) Quantification by densitometry (optical density, OD, mean±s.e.m.) of the corresponding X-ray films. Differences between WT (n=3) versus GRNesCre (n=3) mice were all statistically significant, *P<0.05, **P<0.005, ***P<0.001. (c) Stress-induced activation of the GR in the hippocampus stimulates synapsin-I expression and phosphorylation. Comparison of the expression of synapsin isoforms in wild-type (WT) and GRNesCre mice lacking the expression of GR in neurons, before (t0) and 30 and 120 min after the onset of 30 min of restraint stress. βIII-tubulin was used as a loading control. Nuclear (for GR and Egr-1) and cytoplasmic hippocampal extracts were analyzed by western blot (left panel) and the corresponding X-ray films were quantified by densitometry (right panel, optical density, OD, mean±s.e.m., n=6). *P<0.05; **P<0.005, ***P<0.001, WT versus GRNesCre Newman–Keuls and Duncan post hoc test after ANOVA.
As, under physiological conditions, activation of the GR is mainly obtained during stress, we also analyzed changes in synapsin levels after an acute restraint stress (30 min). For this experiment, blood and hippocampal extracts were collected before stress (t0) and 30 or 120 min after stress onset. As earlier shown in wild-type animals, stress induced the translocation of the GR and the expression of Egr-1.8 In addition, stress also induced an increase in the expression and phosphorylation of pan-synapsin as well of synapsin-Ia/Ib, synapsin-IIa, and synapsin-IIIa (Figure 1c). In GRNesCre mutant mice, lacking the GR, stress-induced expression of Egr-1 was profoundly decreased. In addition, stress-induced increase in phosphorylated synapsin was suppressed and the increase in the expression of synapsin-Ia/Ib was greatly reduced. In contrast, expression of synapsin-IIa and -IIIa were not significantly modified by the absence of the GR (Figure 1c).
These modifications of synapsin and Egr-1 levels were not due to a decrease in stress-induced glucocorticoid secretion in mutant animals. Thus, as has been shown several times,8, 23 basal and stress-induced glucocorticoid levels were higher in GRNesCre mice (Table 1), indicating that the glucocorticoid-negative feedback loop is profoundly impaired in GR mutant mice.
These results indicate that both in basal conditions and after stress, GR positively control the phosphorylation of synapsin and the expression of synapsin-Ia/Ib, but not of other members of this family. Through this double effect, GR may powerfully increase neurotransmitter release. Indeed, the levels of synapsin-Ia/Ib positively control the pool of readily releasable SVs17, 29, 30 and the phosphorylation of synapsin triggers neurotransmitter release.29
Glucocorticoid-induced synapsin-I phosphorylation depends on MAPK activity
In the hippocampus in basal conditions (Figure 1a and b) and after stress (Figure 1c), GR regulate the expression of both Egr-18 and synapsin-I. As GR are phasically activated by the increase in circulating glucocorticoids that occurs during the active phase of the circadian cycle or in response to stress,1, 2, 5 we analyzed whether the acute administration of glucocorticoids would stimulate the expression of synapsin-I. For this experiment, we used the PC12 cell line, which expresses the GR and synapsin-I and -II isoforms.21, 22 In this in vitro model, administration of 10 nM of corticosterone, which replicates the concentration of the hormone induced by stress, increases the expression of Egr-1, the phosphorylation of synapsin, the expression levels of synapsin-I, but not of synapsin-II (Figure 2a).
Corticosterone-induced phosphorylation of synapsin-I involves the MAPK pathway. (a) Total extracts from PC12 cells were analyzed by western blot 1 and 3 h after treatment with 10 nM corticosterone. The corresponding X-ray films were quantified by densitometry (optical density, OD, mean±s.e.m., n=3). *P<0.05; ***P<0.001, in comparison with basal levels. (b) The effects of corticosterone administration (10 nM) on Erk1/2, synapsin-I expressions, and their phosphorylated counterparts were analyzed by western blot in PC12 cells exposed or not to the inhibitor of the MAPK pathway UO126 (10 μM); 1 h after the exposure to 10 nM corticosterone, proteins were extracted and analyzed with relevant antibodies. βIII-tubulin was used as a loading control. (c) Quantification by densitometry (optical density OD, mean±s.e.m., n=4) of the western blot experiments. *P<0.05, **P<0.005, ***P<0.001 in comparison with untreated cells (ANOVA).
Phosphorylation of synapsin has a major function in controlling pre-SV trafficking by inhibiting the dynamic association between the SVs and the actin cytoskeleton.13, 29, 30, 31, 32 We investigated here whether GR-induced activation of the MAPK pathway8 was responsible for the GR-induced phosphorylation of synapsin-I. To test this hypothesis, we studied glucocorticoid-induced phosphorylation of synapsin-I in PC12 cells in the presence of a selective inhibitor of MAPK phosphorylation, UO126. UO126 was used at 10 μM, a dose corresponding to the EC100 of this compound (Figure 2b and c).33
PC12 cells were exposed for 1 h to 10 nM corticosterone in the presence or absence of UO126, after which the proteins were extracted and analyzed by western blot. In the absence of UO126, we found that 1 h exposure to corticosterone induced both phosphorylation of the MAPK pathway (P-Erk1/2) and of synapsin-I. Blocking the phosphorylation of the MAPK pathway almost completely blocked the phosphorylation of synapsin, but not the expression of synapsin-I (Figure 2b and c).
Glucocorticoid-induced expression of synapsin-I depends on Egr-1
In these experiments, we analyzed whether GR increased synapsin-I expression through Egr-1. For this purpose, we silenced Egr-1 expression by using a small-interfering RNA strategy. PC12 cells were transfected with siRNA (-ctrl or -Egr-1) 65 h (t−65) before exposure to 10 nM corticosterone. After 1 h of exposure to corticosterone (t1), the cells were harvested and proteins immediately extracted (Figure 3a). Transfection efficiency was measured by flow cytometry using siRNA-Alexa Fluor 546. With our protocol, it was possible to transfect 74% of PC12 cells with siRNA, with no significant increase in cell death (Figure 3b). Corticosterone increased both Egr-1 and synapsin-I expressions in cells transfected with control siRNA. In contrast, in cells in which Egr-1 expression was silenced, the glucocorticoid-mediated increase in synapsin-I expression was suppressed (Figure 3c and d).
Corticosterone stimulation of synapsin-I expression depends on Egr-1. (a) Schematic illustration of the protocol used to silence Egr-1 expression by using a small-interfering RNA strategy. PC12 cells and hippocampal neurons were transfected with siRNA (-Egr-1, -ctrl, -Alexa Fluor 546, or EGFP); 48 to 65 h later, the cells were exposed to corticosterone (Cort) for 1 h and then proteins were analyzed by western blot (PC12) or immunostaining (neurons). (b) The transfection efficiency in PC12 cells was evaluated by flow cytometry after transfecting siRNA-Alexa Fluor 546. (c) Western blot analyses of the effects of corticosterone administration (10 nM) on synapsin-I and Egr-1 expressions after silencing Egr-1 expression using an siRNA strategy. βIII-tubulin was used as a loading control. (d) Quantification by densitometry (optical density OD, mean±s.e.m., n=3) of the western blot experiments. *P<0.05, **P<0.005, in comparison with untreated cells (ANOVA). (e) Immunostaining of hippocampal neurons cultured for 9 days in vitro showing the sublocalization of the GR proteins (green) in the absence or presence of 100 nM of corticosterone for 1 h. Nuclei were counterstained with DAPI (blue). Merge of the two signals is shown. Scale bar=25 μm. (f) Co-immunolocalization of Egr-1 (green) and synapsin-I (red) proteins on hippocampal neurons cultured for 9 days in vitro in the absence or presence of 100 nM of corticosterone during 1 h. Scale bar=25 μm. (g, h) Quantification of the fluorescence intensity (AU, arbitrary unit) of Egr-1 (g) and synapsin-I (h) -immunoreactive hippocampal neurons in the absence or presence of 100 nM of corticosterone (Cort) during 1 h. (i) After corticosterone treatment, the expression of Egr-1 and synapsin-I proteins in Egr-1-expressing hippocampal neurons increased proportionally. Values shown are mean±s.e.m. **P<0.005, ***P<0.001, in comparison with untreated cells. (j) Immunofluorescence analysis of hippocampal neurons treated with corticosterone (100 nM) for 1 h and earlier co-transfected with EGFP cDNA and siRNA control or siRNA silencing Egr-1 protein. Nuclei were counterstained with DAPI (blue). The white arrows show the mapping of the synapsin-I protein in EGFP-positive hippocampal neurons transfected either with siRNA-ctrl or siRNA-Egr-1. Scale bar=25 μm. (k, l) Quantification of the fluorescence intensity (AU, arbitrary unit) of Egr-1 (k) and synapsin-I (l) -immunoreactive hippocampal neurons transfected with siRNA-ctrl or siRNA-Egr-1 in the presence of 100 nM of corticosterone (Cort) during 1 h. (m) Proportional decrease between Egr-1 and synapsin-I proteins in neurons in which Egr-1 expression was silenced compared with siRNA-ctrl transfection. Values shown are mean±s.e.m. **P<0.005, ***P<0.001, in comparison with siRNA-ctrl transfected cells.
We then analyzed whether these effects could have also been found in primary culture of hippocampal neurons. We also found that, in this cellular context, 1 h of corticosterone treatment promotes the nuclear translocation of the GR proteins (Figure 3e) and a concomitant and proportional increase in both Egr-1 and synapsin-I proteins (Figure 3f–i). Hippocampal neurons were then co-transfected with siRNA (-ctrl or -Egr-1) and EGFP 48 h (t−48) before exposure to 1 h of 100 nM corticosterone. siRNA effects were checked by immunostaining (Figure 3a, j–m). Silencing Egr-1 expression by RNA interference in hippocampal neurons also suppressed the corticosterone-mediated increase in synapsin-I expression (Figure 3j–m). These results confirm that glucocorticoid-induced expression of synapsin-I also depends on Egr-1 in neuronal cells.
In conclusion, these results show that the GR/Egr-1/MAPK cascade controls synapsin-I by complementary mechanisms. Activation of Egr-1 by the GR specifically increases the levels of expression of synapsin-Ia/Ib; in parallel, GR-induced MAPK activation induces the phosphorylation of synapsin-I.
Synapsin-I mediates GR-induced enhancement of fear conditioning
The earlier results suggest that synapsin-I could be one of the molecular effectors through which activation of the GR/Egr-1/MAPK molecular cascade enhances stress-related memories. To test this hypothesis, we analyzed the effects of a modification of the levels of expression of synapsin-I on the retention of fear conditioning.
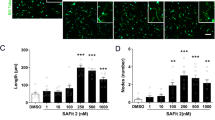
Synapsin-Ia/Ib has recently been shown to be the major Fucα(1–2)Gal glycoprotein in the hippocampus. Inhibiting synapsin-Ia/Ib fucosylation using 2-dGal critically impacts both its cellular half-life expression and turnover in pre-synaptic nerve terminals.24, 34
We, therefore, examined the effects of an intra-hippocampal infusion of 2-dGal both on the expression of synapsin-I and on the enhancement of hippocampal-dependent contextual fear conditioning8, 28 induced by corticosterone (Figure 4). Intra-hippocampal infusion of corticosterone immediately after training increased the conditioned fear induced by an electric shock of low intensity (0.3 mA footshock) inducing a response similar to the one obtained with an electric shock of high intensity (0.7 mA footshock) (Figure 4a). However, the enhancing effect of corticosterone on contextual fear conditioning was completely abolished when 2-dGal (400 mM per side) was infused concomitantly with corticosterone (Figure 4a). To verify the effect of corticosterone and 2-dGal infusion on synapsin-I, a group of animals was killed 2 h after the hippocampal infusions. Hippocampal proteins were then extracted and analyzed by western blot. We found that hippocampal infusion of corticosterone stimulates synapsin-Ia/Ib expression and that this increase was largely blocked by 2-dGal infusion (Figure 4b).
Synapsin-Ia/Ib mediates the enhancement of contextual fear conditioning induced by glucocorticoids. (a) Percentage of conditioned freezing (mean±s.e.m.) displayed by mice re-exposed for 4 min to the conditioning context 24 h after conditioning with either high (0.7 mA footshock; n=12) or low shock intensity (0.3 mA footshock; n=11) and receiving a post-training intra-hippocampal infusion of either vehicle (n=11), 2-dGal (n=11; 400 mM per side), corticosterone (n=12; 10 ng per side), or corticosterone+2-dGal (n=12). (b) Western blot analysis and quantification of synapsin-Ia/Ib hippocampal expression in the presence or absence of the inhibitor of fucosylation 2-dGal (400 mM per side). βIII-tubulin was used as a loading control; 2 h post-hippocampal infusion of 2-dGal, proteins were extracted, analyzed by western blot, and quantified by densitometry (optical density OD, mean±s.e.m., n=4). **P<0.005, ***P<0.0001, in comparison with all the other groups, Newman–Keuls and Scheffe post hoc test after ANOVA.
In conclusion, these results indicate that synapsin-Ia/Ib is one of the effectors through which the GR/Egr-1/MAPK cascade enhances stress-related memory.
Discussion
The central finding of this work is the identification of synapsin-Ia/Ib as an important molecular effector of glucocorticoid-mediated behavioral effects of stress. In particular, synapsin-Ia/Ib seems to be one of the effectors through which the GR/Egr-1/MAPK cascade enhances stress-related memory. Thus, combining in vitro and in vivo approaches, we found that both in basal conditions and after stress, GR, through the activation of Egr-1 expression and MAPK phosphorylation, increase synapsin-Ia/Ib expression and phosphorylation, respectively, and that these events were necessary for the increase in stress-related memories induced by glucocorticoids.
With the results of the present experiment extending those published in an earlier paper, we can single out a complete molecular cascade that is involved in the mediation of stress-related memory (Figure 5). We have earlier shown that stress-induced glucocorticoids increase stress-related memories by activating the GR, which trigger a cascade of events starting with an increase in the expression of the transcription factor Egr-1 and followed by the activation of the MAPK pathway.8 We show here that the activation of Egr-1 by glucocorticoids increases the level of synapsin-I and that the activation of the MAPK pathway induces the phosphorylation of this protein. Silencing Egr-1 expression thus represses the increase in synapsin-Ia/Ib expression induced by glucocorticoids both in PC12 cells and in primary culture of hippocampal neurons. This effect is probably a direct one, as Egr-1 binds to responsive elements in the promoter region of the syn1 gene and is able to activate its transcription in vitro.35, 36, 37 In parallel, mitogen-activated protein kinase (Erk1/2) induces the phosphorylation of synapsin-I, but does not modify its expression. Thus, UO126-mediated blockade of MAPK phosphorylation largely reduced glucocorticoid-induced synapsin-I phosphorylation, but did not change the expression of synapsin-I.
Schematic model of GEMS molecular cascade mediating the enhancement of stress-related memories. During stress, the increase in glucocorticoid secretion (1) activates the glucocorticoid receptors (GR) inducing its nuclear translocation (2). GR trigger the synthesis of the transcription factor Egr-1 (3) and then the activation of the MAPK pathway (4), which further increases Egr-1 production (5). Egr-1 stimulates the transcription of synapsin-Ia/Ib (6), which increases the number of SVs bound to actin and in this way the pool of readily releasable neurotransmitters. Activated MAPK do not modify synapsin-I expression, but induce their phosphorylation (7). Phosphorylated synapsin-Ia/Ib releases SVs bound to actin (8) allowing their transport to the pre-synaptic membrane (9) and neurotransmitter release (10). The double action of GR-induced Egr-1 expression and MAPK activation on both synapsin-I synthesis and phosphorylation can guarantee a sustained increase in neurotransmitter release. Specifically, an increase in glutamate release is likely to mediate the enhancement of stress-related memories that can be blocked both by inhibiting MAPK phosphorylation and synapsin-I expression. This model is based on several studies, including ours, which are discussed in the main text.
Synapsin-I undergoes several cycles of phosphorylation/dephosphorylation on serine residues. In vitro studies have shown that these phosphorylations can involve many different kinases, including cAMP-dependent protein kinase, Ca2+/calmodulin-dependent protein kinase (CaMK) I, CaMK II, CaMK IV, proline-directed kinase, mitogen-activated protein kinase (Erk1/2), cyclin-dependent kinase 1 and 5, tyrosine kinase c-Src, protein kinase C, and p21-activated kinases.13, 14, 30, 38, 39, 40 It is unlikely that in vivo all these kinases phosphorylate synapsin-I at the same time; in all probabilities, their actions depend on the type of stimulation.30 Our results indicate that in vivo the MAPK pathway has an important function in stress-induced phosphorylation of synapsin-I.
Our data also show that the regulation of synapsin-I by the GR/Egr-1/MAPK cascade increases stress-related memory. Inhibition of synapsin-I expression by 2-dGal thus abolished glucocorticoid-induced increase in contextual fear conditioning. These results extend earlier knowledge and start clarifying the function of different synapsin isoforms in memory formation. Thus, it has been earlier shown that synapsin-deficient Drosophila melanogaster flies display a deficit in learning and memory,20, 41 and synapsin-I null mice display an increase in age-dependent cognitive impairments.42 In parallel, the learning of a spatial memory task increases expression of synapsin-I in the hippocampus,19, 43 and transgenic mice expressing a constitutively active form of H-ras (H-rasG12V) exhibit increased Erk-dependent phosphorylation of synapsin-I and dramatic enhancements in hippocampus-dependent learning that can be reversed by the deletion of synapsin-I.44 Our data indicate that synapsin-Ia/Ib have an important function in the formation of hippocampal-mediated stress-related memory.
As all the synapsin isoforms (-I, -II, and -III) are expressed within the hippocampus,14, 45 it could be argued that other synapsins could be modified by 2-dGal and consequently be involved in the modulation of fear memory. In fact, synapsin fucosylation sites are localized within the D domain of synapsin, a region that is found only in the synapsin-Ia/Ib isoforms and not in the other isoforms.14 In addition, western blot analysis of adult hippocampal lysates from synapsin-I-deficient mice revealed a loss of the fucosylated bands, confirming that synapsins Ia and Ib are the predominant Fucα(1–2)Gal glycoproteins in the hippocampus.24
The inhibition of stress-related memory by blocking synapsin-I fucosylation also supports growing evidence, indicating that carbohydrate post-translational modifications to N- and O-linked glycoproteins could participate in cognitive processes such as learning and memory46, 47 and in synaptic plasticity.34, 48, 49
The GEMS cascade enhances stress-related memory very likely by increasing the release of neurotransmitters. Unphosphorylated synapsins bind SVs tightly to actin. These synapsin-actin-bound SVs constitute the readily releasable pool of vesicles that is positively regulated by synapsin-I levels.50 Phosphorylation of synapsin-I then releases SVs from the synapsin-actin-bound state,31 which in turn allows their transport to the sites of exocytosis and the release of neurotransmitters.29, 30 Consequently, the increase in the level of synapsin-I induced by GR-induced Egr-1 will increase the stock of neurotransmitters readily releasable, and its phosphorylation by GR-activated MAPK triggers its actual release.
Several lines of evidences suggest that the enhancement of stress-related memory mediated by the GEMS pathway is mediated by glutamate release. First, in the hippocampus, synapsin-I is exclusively expressed in glutamatergic pyramidal neurons, but is absent from GABA interneurons.51, 52, 53 Second, intra-peritoneal administration of corticosterone increases glutamate, but not GABA release in the hippocampus.54 This effect depends on GR activation, as it can be prevented by pre-treatment with the GR antagonist mifepristone.55 Finally, synapsin-I has been shown to regulate glutamate release from SVs17 through a synaptic activity-dependent mechanism56 that is impaired in mice lacking the synapsin-I gene.16, 17, 57 An increase in glutamate release triggered by the GEMS pathway can in turn modify synaptic plasticity, which is probably involved in the enhancement of stress-related memory.18
In conclusion, our data provide a complete molecular pathway GEMS (Figure 5) through which glucocorticoids can enhance the memory of stressful events,5, 6, 7, 8 and reveal synapsin-Ia/Ib as an important molecular effector of the behavioral consequences of stress.
References
McEwen BS . Effects of adverse experiences for brain structure and function. Biol Psychiatry 2000; 48: 721–731.
De Kloet ER . Hormones, brain and stress. Endocr Regul 2003; 37: 51–68.
Piazza PV, Le Moal M . Glucocorticoids as a biological substrate of reward: physiological and pathophysiological implications. Brain Res Brain Res Rev 1997; 25: 359–372.
Sapolsky RM . Glucocorticoids and hippocampal atrophy in neuropsychiatric disorders. Arch Gen Psychiatry 2000; 57: 925–935.
De Kloet ER, Oitzl MS, Joels M . Stress and cognition: are corticosteroids good or bad guys? Trends Neurosci 1999; 22: 422–426.
McGaugh JL, Roozendaal B . Role of adrenal stress hormones in forming lasting memories in the brain. Curr Opin Neurobiol 2002; 12: 205–210.
Oitzl MS, Reichardt HM, Joels M, De Kloet ER . Point mutation in the mouse glucocorticoid receptor preventing DNA binding impairs spatial memory. Proc Natl Acad Sci USA 2001; 98: 12790–12795.
Revest JM, Di Blasi F, Kitchener P, Rouge-Pont F, Desmedt A, Turiault M et al. The MAPK pathway and Egr-1 mediate stress-related behavioral effects of glucocorticoids. Nat Neurosci 2005; 8: 664–672.
Holsboer F . The corticosteroid receptor hypothesis of depression. Neuropsychopharmacology 2000; 23: 477–501.
Wei Q, Lu XY, Liu L, Schafer G, Shieh KR, Burke S et al. Glucocorticoid receptor overexpression in forebrain: a mouse model of increased emotional lability. Proc Natl Acad Sci USA 2004; 101: 11851–11856.
Funder JW . Glucocorticoid receptors. J Steroid Biochem Mol Biol 1992; 43: 389–394.
Tronche F, Kellendonk C, Reichardt HM, Schutz G . Genetic dissection of glucocorticoid receptor function in mice. Curr Opin Genet Dev 1998; 8: 532–538.
Evergren E, Benfenati F, Shupliakov O . The synapsin cycle: a view from the synaptic endocytic zone. J Neurosci Res 2007; 85: 2648–2656.
Valtorta F, Benfenati F, Greengard P . Structure and function of the synapsins. J Biol Chem 1992; 267: 7195–7198.
Ferreira A, Rapoport M . The synapsins: beyond the regulation of neurotransmitter release. Cell Mol Life Sci 2002; 59: 589–595.
Jovanovic JN, Czernik AJ, Fienberg AA, Greengard P, Sihra TS . Synapsins as mediators of BDNF-enhanced neurotransmitter release. Nat Neurosci 2000; 3: 323–329.
Nichols RA, Chilcote TJ, Czernik AJ, Greengard P . Synapsin I regulates glutamate release from rat brain synaptosomes. J Neurochem 1992; 58: 783–785.
Howland JG, Wang YT . Synaptic plasticity in learning and memory: stress effects in the hippocampus. Prog Brain Res 2008; 169: 145–158.
John JP, Sunyer B, Hoger H, Pollak A, Lubec G . Hippocampal synapsin isoform levels are linked to spatial memory enhancement by SGS742. Hippocampus 2009; 19: 731–738.
Godenschwege TA, Reisch D, Diegelmann S, Eberle K, Funk N, Heisenberg M et al. Flies lacking all synapsins are unexpectedly healthy but are impaired in complex behaviour. Eur J Neurosci 2004; 20: 611–622.
Carpentier R, Sacchetti P, Segard P, Staels B, LeFebvre P . The glucocorticoid receptor is a co-regulator of the orphan nuclear receptor Nurr1. J Neurochem 2008; 104: 777–789.
Das KP, Freudenrich TM, Mundy WR . Assessment of PC12 cell differentiation and neurite growth: a comparison of morphological and neurochemical measures. Neurotoxicol Teratol 2004; 26: 397–406.
Tronche F, Kellendonk C, Kretz O, Gass P, Anlag K, Orban PC et al. Disruption of the glucocorticoid receptor gene in the nervous system results in reduced anxiety. Nat Genet 1999; 23: 99–103.
Murrey HE, Gama CI, Kalovidouris SA, Luo WI, Driggers EM, Porton B et al. Protein fucosylation regulates synapsin Ia/Ib expression and neuronal morphology in primary hippocampal neurons. Proc Natl Acad Sci USA 2006; 103: 21–26.
Banker GA, Cowan WM . Rat hippocampal neurons in dispersed cell culture. Brain Res 1977; 126: 397–342.
Bottenstein JE, Sato GH . Growth of a rat neuroblastoma cell line in serum-free supplemented medium. Proc Natl Acad Sci USA 1979; 76: 514–517.
Kitchener P, Di Blasi F, Borrelli E, Piazza PV . Differences between brain structures in nuclear translocation and DNA binding of the glucocorticoid receptor during stress and the circadian cycle. Eur J Neurosci 2004; 19: 1837–1846.
Desmedt A, Garcia R, Jaffard R . Differential modulation of changes in hippocampal-septal synaptic excitability by the amygdala as a function of either elemental or contextual fear conditioning in mice. J Neurosci 1998; 18: 480–487.
Hosaka M, Hammer RE, Sudhof TC . A phospho-switch controls the dynamic association of synapsins with synaptic vesicles. Neuron 1999; 24: 377–387.
Yamagata Y . New aspects of neurotransmitter release and exocytosis: dynamic and differential regulation of synapsin I phosphorylation by acute neuronal excitation in vivo. J Pharmacol Sci 2003; 93: 22–29.
Jovanovic JN, Benfenati F, Siow YL, Sihra TS, Sanghera JS, Pelech SL et al. Neurotrophins stimulate phosphorylation of synapsin I by MAP kinase and regulate synapsin I-actin interactions. Proc Natl Acad Sci USA 1996; 93: 3679–3683.
Turner KM, Burgoyne RD, Morgan A . Protein phosphorylation and the regulation of synaptic membrane traffic. Trends Neurosci 1999; 22: 459–464.
Favata MF, Horiuchi KY, Manos EJ, Daulerio AJ, Stradley DA, Feeser WS et al. Identification of a novel inhibitor of mitogen-activated protein kinase. J Biol Chem 1998; 273: 18623–18632.
Murrey HE, Hsieh-Wilson LC . The chemical neurobiology of carbohydrates. Chem Rev 2008; 108: 1708–1731.
Al Sarraj A, Day RM, Thiel G . Specificity of transcriptional regulation by the zinc finger transcription factors Sp1, Sp3, and Egr-1. J Cell Biochem 2005; 94: 153–167.
James AB, Conway AM, Thiel G, Morris BJ . Egr-1 modulation of synapsin I expression: permissive effect of forskolin via cAMP. Cell Signal 2004; 16: 1355–1362.
Thiel G, Schoch S, Petersohn D . Regulation of synapsin I gene expression by the zinc finger transcription factor zif268/egr-1. J Biol Chem 1994; 269: 15294–15301.
Browning MD, Dudek EM . Activators of protein kinase C increase the phosphorylation of the synapsins at sites phosphorylated by cAMP-dependent and Ca2+/calmodulin-dependent protein kinase in the rat hippocampal slice. Synapse 1992; 10: 62–70.
Sakurada K, Kato H, Nagumo H, Hiraoka H, Furuya K, Ikuhara T et al. Synapsin I is phosphorylated at Ser603 by p21-activated kinases (PAKs) in vitro and in PC12 cells stimulated with bradykinin. J Biol Chem 2002; 277: 45473–45479.
Severin Jr SE, Moskvitina EL, Bykova EV, Lutzenko SV, Shvets VI . Synapsin I from human brain. Phosphorylation by Ca2+, phospholipid-dependent protein kinase. FEBS Lett 1989; 258: 223–226.
Michels B, Diegelmann S, Tanimoto H, Schwenkert I, Buchner E, Gerber B . A role for synapsin in associative learning: the Drosophila larva as a study case. Learn Mem 2005; 12: 224–231.
Corradi A, Zanardi A, Giacomini C, Onofri F, Valtorta F, Zoli M et al. Synapsin-I- and synapsin-II-null mice display an increased age-dependent cognitive impairment. J Cell Sci 2008; 121: 3042–3051.
Gomez-Pinilla F, So V, Kesslak JP . Spatial learning induces neurotrophin receptor and synapsin I in the hippocampus. Brain Res 2001; 904: 13–19.
Kushner SA, Elgersma Y, Murphy GG, Jaarsma D, van Woerden GM, Hojjati MR et al. Modulation of presynaptic plasticity and learning by the H-ras/extracellular signal-regulated kinase/synapsin I signaling pathway. J Neurosci 2005; 25: 9721–9734.
Kao HT, Porton B, Czernik AJ, Feng J, Yiu G, Haring M et al. A third member of the synapsin gene family. Proc Natl Acad Sci USA 1998; 95: 4667–4672.
Lorenzini CG, Baldi E, Bucherelli C, Sacchetti B, Tassoni G . 2-Deoxy-D-galactose effects on passive avoidance memorization in the rat. Neurobiol Learn Mem 1997; 68: 317–324.
Rose SP, Jork R . Long-term memory formation in chicks is blocked by 2-deoxygalactose, a fucose analog. Behav Neural Biol 1987; 48: 246–258.
Tallent MK, Varghis N, Skorobogatko Y, Hernandez-Cuebas L, Whelan K, Vocadlo DJ et al. In vivo modulation of O-GlcNAc levels regulates hippocampal synaptic plasticity through interplay with phosphorylation. J Biol Chem 2009; 284: 174–181.
Krug M, Jork R, Reymann K, Wagner M, Matthies H . The amnesic substance 2-deoxy-D-galactose suppresses the maintenance of hippocampal LTP. Brain Res 1991; 540: 237–242.
Baldelli P, Fassio A, Valtorta F, Benfenati F . Lack of synapsin I reduces the readily releasable pool of synaptic vesicles at central inhibitory synapses. J Neurosci 2007; 27: 13520–13531.
Bogen IL, Boulland JL, Mariussen E, Wright MS, Fonnum F, Kao HT et al. Absence of synapsin I and II is accompanied by decreases in vesicular transport of specific neurotransmitters. J Neurochem 2006; 96: 1458–1466.
Bogen IL, Jensen V, Hvalby O, Walaas SI . Synapsin-dependent development of glutamatergic synaptic vesicles and presynaptic plasticity in postnatal mouse brain. Neuroscience 2009; 158: 231–241.
Melloni Jr RH, Hemmendinger LM, Hamos JE, DeGennaro LJ . Synapsin I gene expression in the adult rat brain with comparative analysis of mRNA and protein in the hippocampus. J Comp Neurol 1993; 327: 507–520.
Venero C, Borrell J . Rapid glucocorticoid effects on excitatory amino acid levels in the hippocampus: a microdialysis study in freely moving rats. Eur J Neurosci 1999; 11: 2465–2473.
Wang CC, Wang SJ . Modulation of presynaptic glucocorticoid receptors on glutamate release from rat hippocampal nerve terminals. Synapse 2009; 63: 745–751.
Chi P, Greengard P, Ryan TA . Synaptic vesicle mobilization is regulated by distinct synapsin I phosphorylation pathways at different frequencies. Neuron 2003; 38: 69–78.
Li L, Chin LS, Shupliakov O, Brodin L, Sihra TS, Hvalby O et al. Impairment of synaptic vesicle clustering and of synaptic transmission, and increased seizure propensity, in synapsin I-deficient mice. Proc Natl Acad Sci USA 1995; 92: 9235–9239.
Acknowledgements
We thank Drs D Choquet and O Thoumine for invaluable help with the experiments using the hippocampal cultures. We acknowledge the technical support of M Petit, C Breillat, B Teissier, and D Bouchet. The microscopy was performed in the Bordeaux Imaging Center of the University of Bordeaux II. The help of P Legros, C Poujol, and L Malicieux is acknowledged. This work was supported by INSERM, Agence Nationale pour la Recherche (ANR, contract HICOMET), Bordeaux Institute for Neurosciences (IFR8), the University Victor Segalen-Bordeaux2, and Conseil Régional d’Aquitaine, the 6th Framework European Programme (PHECOMP, contract no. FP6-037669).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
This work is licensed under the Creative Commons Attribution-NonCommercial-No Derivative Works 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/3.0/
About this article
Cite this article
Revest, JM., Kaouane, N., Mondin, M. et al. The enhancement of stress-related memory by glucocorticoids depends on synapsin-Ia/Ib. Mol Psychiatry 15, 1140–1151 (2010). https://doi.org/10.1038/mp.2010.40
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/mp.2010.40
Keywords
This article is cited by
-
PAI-1 protein is a key molecular effector in the transition from normal to PTSD-like fear memory
Molecular Psychiatry (2021)
-
Glucocorticoid Receptor Activation Restores Learning Memory by Modulating Hippocampal Plasticity in a Mouse Model of Brain Vitamin B12 Deficiency
Molecular Neurobiology (2021)
-
Two New Fucose-α (1–2)-Glycans Assigned In The Healthy Human Brain Taking The Number To Seven
Scientific Reports (2019)
-
In Vivo and In Vitro Neuronal Plasticity Modulation by Epigenetic Regulators
Journal of Molecular Neuroscience (2018)
-
A novel non genomic glucocorticoid signaling mediated by a membrane palmitoylated glucocorticoid receptor cross talks with GnRH in gonadotrope cells
Scientific Reports (2017)