Abstract
Women who test positive for a high-risk type of the human papillomavirus (HPV) require triage testing to identify those women with cervical intraepithelial neoplasia grade 3 or cancer (≥CIN3). Although Pap cytology is considered an attractive triage test, its applicability is hampered by its subjective nature. This study prospectively compared the clinical performance of p16/Ki-67 dual-stained cytology to that of Pap cytology, with or without HPV16/18 genotyping, in high-risk HPV-positive women visiting gynecologic outpatient clinics (n=446 and age 18–66 years). From all women, cervical scrapes (for Pap cytology, HPV16/18 genotyping, and p16/Ki-67 dual-stained cytology) and colposcopy-directed biopsies were obtained. The sensitivity of p16/Ki-67 dual-stained cytology for ≥CIN3 (93.8%) did neither differ significantly from that of Pap cytology (87.7%; ratio 1.07 and 95% confidence interval (CI): 0.97–1.18) nor from that of Pap cytology combined with HPV16/18 genotyping (95.1%; ratio 0.99 and 95% CI: 0.91–1.07). However, the specificity of p16/Ki-67 dual-stained cytology for ≥CIN3 (51.2%) was significantly higher than that of Pap cytology (44.9%; ratio 1.14 and 95% CI: 1.01–1.29) and Pap cytology combined with HPV16/18 genotyping (25.8%; ratio 1.99 and 95% CI: 1.68–2.35). After exclusion of women who had been referred because of abnormal Pap cytology, the specificity of p16/Ki-67 dual-stained cytology for ≥CIN3 (56.7%) remained the same, whereas that of Pap cytology (60.3%) increased substantially, resulting in a similar specificity of both assays (ratio 0.94 and 95% CI: 0.83–1.07) in this sub-cohort. In summary, p16/Ki-67 dual-stained cytology has a good clinical performance and is an interesting objective microscopy-based triage tool for high-risk HPV-positive women.
Similar content being viewed by others
Main
Persistent infection with a high-risk type of the human papillomavirus (HPV) is essential for the development of almost all cervical cancers.1, 2 Testing for high-risk HPV has been shown to provide superior protection against cervical intraepithelial neoplasia grade 3 and cervical cancer (together referred to as ≥CIN3) compared with cervical cytology (Pap cytology).3 However, most women who test positive for high-risk HPV clear the virus spontaneously and do not develop clinically relevant cervical disease. Therefore, additional triage testing is required to identify the subgroup of high-risk HPV-positive women who actually have ≥CIN3, thereby reducing the risk of overdiagnosis, unnecessary colposcopy referral, and treatment.
Pap cytology is considered an effective triage strategy for high-risk HPV-positive women.4, 5 Yet, the performance of Pap cytology as a triage test is limited by its subjective nature and thus its dependence on a high level of expertise.4, 5 In search of a reproducible, objective, and direct triage method with a higher sensitivity, several molecular assays have been proposed as valuable additions to Pap cytology. Among them is the assessment of the presence of HPV16 and/or HPV18 (HPV16/18 genotyping), a method to identify women with the most carcinogenic HPV types that together account for the majority of ≥CIN3.6, 7, 8 The combined use of Pap cytology and HPV16/18 genotyping yields a substantially lower ≥CIN3 risk after a negative test result compared with Pap cytology alone. However, this is at the cost of a lower specificity, leading to unnecessarily high referral rates.4, 5, 7
Another triage test that has been described as a promising alternative to Pap cytology is the combined p16 and Ki-67 immunostaining of cervical cytology specimens. Simultaneous co-expression of the anti-proliferative p16 protein and the proliferation marker Ki-67 in the same cervical epithelial cell is a biomarker combination indicative of high-risk HPV-induced cell cycle deregulation and transforming HPV infection.9, 10 The use of p16/Ki-67 dual-stained cytology has been shown to yield a high specificity for ≥CIN3.11, 12, 13, 14 In addition, p16/Ki-67 dual-stained cytology has been reported to decrease the inter-observer variability of cytology scoring.15
In this study, we compared the performance of p16/Ki-67 dual-stained cytology with that of Pap cytology, with or without HPV16/8 genotyping, on cervical liquid-based cytology specimens for the detection of ≥CIN3 in high-risk HPV-positive women from a gynecologic outpatient population.
Materials and methods
Study Design, Participants, and Procedures
The present study was conducted within the COMETH study, of which the design, participants, and procedures have been previously described in detail.16 From December 2010 till December 2013, women aged 18–70 years were asked to participate in a prospective observational multi-center cohort study during their visit to the gynecologic outpatient clinics of six hospitals in The Netherlands. The study was approved by the Medical Ethical Committee of all participating hospitals (METc-VUmc2009/178) and registered in the Dutch National Trial Registry (NTR2447). Women with a history of treatment for cervical dysplasia or cancer, current cancer, pregnancy, or lactation were excluded from participation.16 Women could participate in the study regardless of their reason for referral to the gynecology outpatient clinic. After providing informed consent, participants collected cervico-vaginal lavage material (using a Delphi screener, Delphi Bioscience, The Netherlands) for high-risk HPV testing. Women who were eligible for the study and tested positive for high-risk HPV on the cervico-vaginal lavage were invited for a physician-taken cervical scrape and a colposcopy.16 Cervical scrapes were stored in Thinprep PreservCyt solution (Hologic, USA). Each cervical liquid-based cytology sample was used to perform a high-risk HPV test with subsequent HPV16/18 genotyping, to prepare one slide for Pap cytology testing and one separate cytology slide for p16/Ki-67 dual-stained cytology testing. Additional aliquots have been removed for other molecular tests (as described recently)16 before the vials have been used to prepare slides for p16/Ki-67 dual-stained cytology testing.
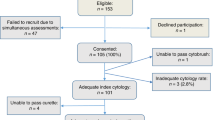
Figure 1 shows the composition of the study population. As described before,16 among a total of 2970 women who gave informed consent and participated in the study, 717 women (24%) tested positive for high-risk HPV on self-collected material. After exclusion of 78 women (11%) for various reasons described in Figure 1, cervical scrapes were obtained from the remaining 639 women. Cervical scrapes that tested positive for high-risk HPV and contained sufficient liquid-based cytology material (n=535) were tested for Pap cytology, p16/Ki-67 dual-stained cytology, and HPV16/18 genotyping. No invalid HPV16/18 genotyping results were recorded. Cases with invalid test results for Pap cytology (6/535; 1%) or p16/Ki-67 dual-stained cytology (88/535; 16%) were excluded from further analyses. The remaining cohort comprised the final study population (n=446; ages 18–66 years). All of these women had a valid histological endpoint obtained by colposcopy-directed biopsy.
In the final study population, 44% (197/446) of women had been referred to the gynecologist because of a recent abnormal Pap cytology result. The remaining women (56%; 249/446) were visiting the gynecologist for other, non-cervix-related gynecologic complaints. For logistic reasons, 44% (196/446) of cervical scrapes were obtained directly before colposcopy, whereas the remaining scrapes (56%; 250/446) were collected in a separate visit 2–3 weeks before colposcopy.
HPV Testing and Genotyping
DNA isolation of 1/10th of the liquid-based cytology material was performed with the Nucleo-Spin 96 Tissue kit (Macherey-Nagel, Germany) and a Microlab Star robotic system (Hamilton, Germany) according to manufacturers’ instructions.17 The isolated DNA was subjected to general primers 5+/6+ PCR–enzyme immunoassay (GP5+/6+; Diassay, The Netherlands).18 A microsphere bead-based assay (Luminex)19 was used for genotyping of the high-risk HPV types 16/18/31/33/35/39/45/51/52/56/58/59/66/68.
Pap cytology
For Pap cytology testing, liquid-based cytology preparations were processed using a Thinprep 5000 processor, Pap stained, and cytologically classified according to the CISOE-A classification (reporting on Composition, pthe resence or absence of Inflammation, grading Squamous-, Other- or Endometrial-, and endocervical (columnar) atypia with a separate score for Adequacy) used in The Netherlands as previously described.16, 20 CISOE-A results were translated into the Bethesda classification,21 in which borderline or mild dyskaryosis equals atypical squamous cells of undetermined significance (ASC-US)/ASCs, cannot exclude high-grade squamous intraepithelial lesion (ASC-H)/low-grade squamous intraepithelial lesion (LSIL), and worse than borderline or mild dyskariosis equals high-grade squamous intraepithelial lesion. Cytotechnicians were aware of the high-risk HPV-positive status of the cervical scrapes but not of the results of high-risk HPV genotyping.
p16/Ki-67 Dual-Stained Cytology
After HPV testing, Pap cytology testing, and the removal of aliquots for other molecular tests,16 an additional cytology slide was produced from each liquid-based cytology sample using a Thinprep 5000 processor (Hologic). For p16/Ki-67 dual-staining, a commercial kit specifically designed for simultaneous detection of p16 and Ki-67 in cervical cytology samples was used (CINtecPlus, Roche mtm Laboratories, Germany) according to the instructions of the manufacturer, as described previously.22, 23 Slides were analyzed and scored by an experienced cytotechnologist, who was blinded to all other study data but who was aware of high-risk HPV-positive status of the cervical specimens. Samples were considered p16/Ki-67 dual-stain positive when immunoreactivity for both p16 and Ki-67 was detected within the same cell (that is, a cytoplasmic brown staining for p16, together with a nuclear red staining for Ki-67),22 in at least one cell per slide.
Statistical Analysis
The sample size was set such that 90% power was achieved for demonstrating non-inferiority of p16/Ki-67 dual-stained cytology or HPV16/18 genotyping compared with Pap cytology using a matched-sample score test.24, 25 A minimum of 300 high-risk HPV-positive women needed to be included at a rejection rate α of 0.05. Histologically confirmed ≥CIN3 was used as primary study endpoint. ≥CIN2 was used as secondary study endpoint, as the category of CIN2 reflects a heterogeneous disease, of which a substantial part results from productive high-risk HPV infections1 and regresses spontaneously.26, 27 The study endpoint was based on the histological outcome of the colposcopy-directed biopsy or, if classified worse, on the histology result of the specimen excised by LLETZ, conization, or hysterectomy. For Pap cytology, Pap cytology combined with HPV16/18 genotyping and p16/Ki-67 dual-stained cytology, sensitivity, specificity, positive predictive value, and the complemented negative predictive value (a measure of disease risk after a negative result) for the detection of ≥CIN3 and ≥CIN2 were calculated with 95% confidence intervals (95% CIs). To clarify the attribution of HPV16/18 genotyping to the combination of Pap cytology with HPV16/18 genotyping, data were also presented for HPV16/18 genotyping alone. In concordance with earlier work on this population,16 relative sensitivities (ratios of the sensitivity of one test to the sensitivity of another test) and relative specificities (ratios of the specificity of one test to the specificity of another test) were calculated with 95% CIs, to enable comparisons. If the 95% CIs of the relative sensitivity or specificity was entirely below or above 1, this difference in sensitivity or specificity was considered significant. In case such a significant difference was not found, an additional non-inferiority test was performed. Non-inferiority was defined as a relative sensitivity or specificity of at least 90% using a matched-sample score test.24, 25 We considered three factors that might influence sensitivity and specificity of the described tests. First, the age of the participants (aged ≥30 years vs <30 years); second, the reason of referral to the gynecologist (non-cervix-related gynecologic complaints vs a recent abnormal cytology result in cervical screening); and third, the moment that the cervical scrape was collected (during a separate visit before colposcopy vs combined with the colposcopy procedure in one visit). We used logistic regression to study the influence of these factors. After finding a factor that significantly influenced the performance of the different tests (significance: P<0.05), we performed a subgroup analysis after stratification for this factor. All statistical analyses were performed in IBM SPSS Statistics 20 and STATA 11.0.
Results
Test Positivity and Histological Endpoints
Cytology was abnormal (borderline or mild dyskariosis or worse) in 61% (272/446), p16/Ki-67 dual-stained cytology was positive in 57% (254/446) of women, and 50% (221/446) women tested positive for HPV16 and/or HPV18 (HPV16/18); 78% (348/446) of women tested positive for cytology and/or HPV16/18.
Two (0.4%) women were diagnosed with cervical carcinoma (one adenosquamous carcinoma and one squamous cell carcinoma), 79 women (18%) had CIN3, 85 women (19%) had CIN2, 122 women (27%) had CIN1, and 158 women (35%) had no CIN.
Performance of Triage Tests in the Total Study Population
Test specifications of Pap cytology, HPV16/18 genotyping, Pap cytology combined with HPV16/18 genotyping, and p16/Ki-67 dual-stained cytology, for the detection of ≥CIN3 and ≥CIN2, are shown in Table 1. The ≥CIN3 sensitivity of p16/Ki-67 dual-stained cytology was 93.8%, which did not differ significantly from that of Pap cytology (87.7%; ratio 1.07 and 95% CI: 0.97–1.18) or Pap cytology combined with HPV16/18 genotyping (95.1%; ratio 0.99 and 95% CI: 0.91–1.07). The ≥CIN3 specificity of p16/Ki-67 dual-stained cytology (51.2%) was significantly higher than that of Pap cytology (44.9%; ratio 1.14 and 95% CI: 1.01–1.29), and that of Pap cytology combined with HPV16/18 genotyping (25.8%; ratio 1.99 and 95% CI: 1.68–2.35). The complemented negative predictive values for ≥CIN3 of p16/Ki-67 dual-stained cytology, Pap cytology, and Pap cytology combined with HPV16/18 genotyping were 2.6% (95% CI: 0.4–4.9%), 5.7% (95% CI: 2.3–9.2%) and 4.1% (95% CI: 0.2–8.0%), respectively.
Factors Potentially Influencing Test Performance
Three factors were evaluated for a potential influence on test performance: (1) age of the participants, ie, women ≥30 years (n=254) or <30 years (n=192); (2) reason of referral, ie, because of a recent abnormal cytological scrape (n=197) or non-cervix-related gynecologic complaints (n=249); (3) moment of taking the cervical scrape, ie, at a separate visit 2–3 weeks before the colposcopy visit (n=250) or at the same visit as colposcopy (n=196).
As shown in Table 2, only ≥CIN3 specificity of Pap cytology was significantly influenced by the referral reason of the participant; ≥CIN3 specificity of Pap cytology was significantly lower in women referred for abnormal cytology than in women referred for other non-cervix-related complaints. In multivariate analyses, the performance of Pap cytology was neither influenced by age nor by the sampling moment of the cervical scrape (Table 2). Both sensitivity and specificity of HPV16/18 genotyping and p16/Ki-67 dual-stained cytology were independent of the age of the participant, referral reason of the participant, and the point in time when the cervical scrape was collected (data not shown).
Subgroup Analysis of Test Performance
Given the significant influence of referral reason of the participant on the ≥CIN3 specificity of Pap cytology, we performed additional analyses after exclusion of women who were referred for abnormal cytology (n=197), leaving only women who were referred for other non-cervix-related complaints (n=249). Histological endpoints for this subgroup are shown in Figure 1. In this subgroup, ≥CIN3 specificity of Pap cytology (60.3%; 95% CI: 53.9–66.7%) and Pap cytology combined with HPV16/18 genotyping (33.9%; 95% CI: 27.7–40.1%) were significantly higher than in the total study population (44.9 and 25.8% respectively; Table 1). The ≥CIN3 specificity of p16/Ki-67 dual-stained cytology (56.7%; 95% CI: 50.2–63.2%) in this subgroup was similar to its specificity in the total study population (51.2%). In this subgroup, the ≥CIN3 specificity of p16/Ki-67 dual-stained cytology (56.7%) did not differ significantly from that of cytology (60.3%; ratio 0.94 and 95% CI: 0.83–1.07). Similar to analyses in the total study population, p16/Ki-67 dual-stained cytology had a higher ≥CIN3 specificity compared with Pap cytology combined with HPV16/18 genotyping (56.7 vs 33.9%; ratio 1.67 and 95% CI: 1.40–1.99).
≥CIN3 Detection by Different Tests
Table 3 shows the combinations of results for Pap cytology, HPV16/18 genotyping, and p16/Ki-67 dual-stained cytology in all women with ≥CIN3. A majority of women with ≥CIN3 (54/81; 67%), including one woman with an adenosquamous carcinoma, were positive for all three tests. In another 16.0% (13/81) of ≥CIN3 cases, including one squamous cell carcinoma, both Pap cytology and p16/Ki-67 dual-stained cytology were positive, whereas HPV16/18 genotyping was negative (the squamous cell carcinoma harbored HPV39). Among 10 Pap cytology-negative CIN3 cases (12%), 9 (11% of all ≥CIN3 cases) tested positive for p16/Ki-67 dual-stained cytology, including 5 cases (6% of all ≥CIN3 cases) that were also HPV16 and/or 18 positive. Among five (6% of all ≥CIN3 cases) p16/Ki-67 dual-stained cytology-negative CIN3 cases, four (5% of all ≥CIN3 cases) tested Pap cytology-positive and one (1% of all ≥CIN3 cases) was only detected by HPV16/18 genotyping.
Discussion
In the present study, comprising high-risk HPV-positive women of a gynecologic outpatient population, the sensitivity of p16/Ki-67 dual-stained cytology for the detection of ≥CIN3 was similar to that of Pap cytology. In the total study population, p16/Ki-67 dual-stained cytology revealed a higher specificity for ≥CIN3 than Pap cytology. After exclusion of women who had been referred because of abnormal cytology, the ≥CIN3 specificity of p16/Ki-67 dual-stained cytology remained the same, whereas that of Pap cytology increased substantially, resulting in a similar ≥CIN3 specificity of both assays in this particular subgroup of women. p16/Ki-67 dual-stained cytology also yielded similar ≥CIN3 sensitivities to the combination of Pap cytology with HPV16/18 genotyping, at a significantly higher ≥CIN3 specificity. The complementarity of the evaluated tests for ≥CIN3 detection was limited.
In search of a tool to identify women in need of treatment among high-risk HPV-positive women, the subjective nature and related limited reproducibility of cervical cytology necessitate the exploration of more objective tests. In previous studies, p16/Ki-67 dual-stained cytology has been evaluated as a primary cervical screening test,28 as a triage test for women with low-grade cervical cytology23, 29 or for HPV-positive women with normal cytology,22 and in colposcopy referral populations.13, 15, 30 A recent large study on the performance of p16/Ki-67 dual-stained cytology as a triage marker for high-risk HPV-positive women found that p16/Ki-67 dual-stained cytology outperformed Pap cytology with regard to ≥CIN3 specificity, but had a comparable ≥CIN3 sensitivity.31 Our study involved a different study population and we also found a specificity advantage of p16/Ki-67 dual-stained cytology when taking into account the total study population. In our study, this specificity advantage could be attributed to the subgroup of women referred for abnormal cytology, in which a significantly lower ≥CIN3 specificity of Pap cytology, but not of p16/Ki-67 dual-stained cytology, was found.
The sensitivity of cytology in this study was relatively high. Nonetheless, in this outpatient cohort some women with CIN3 were missed by cytology. Retrospective informed cytology revision of the cytology-negative CIN3 cases showed that four CIN3 cases were initially missed by cytology (three re-classified as ASC-US and one LSIL), whereas five were again classified as cytology negative during revision. Besides the use of an outpatient population, the prior knowledge of high-risk HPV presence to the cytotechnicians and cytopathologists might be an explanation for the high sensitivity of cytology in the current study.32, 33 By adjusting the threshold of abnormal cytology from ≥ASC-US to ≥LSIL in our study, sensitivity would decrease from 87.7 to 77.8%, with a corresponding increase in specificity from 44.9 to 72.3%. A similar finding was recently described by Ebisch et al. (in press).
Strengths of the present study include its sample size, the large age range of the population (18–66 years), and the presence of a histological endpoint for each participant. Moreover, our results further substantiate previous work31 in a different geographical region and population.
It should be kept in mind that this study was performed in an outpatient population with a relatively high ≥CIN3 prevalence, which limits the direct translatability of our results into screening settings. In addition, this study was limited by a relatively high number of cytological slides that were non-evaluable for p16/Ki-67 dual staining (16%; 88/535), mainly due to insufficient cellularity (72%; 64/88). Of note, during a retrospective informed revision, all CIN3 cases with negative p16/Ki-67 dual-stained cytology (n=4) were confirmed as p16/Ki-67 dual-stain negative, owing to a low cellularity. The high number of hypocellular slides might result from the secondary production of a cytological slide for p16/Ki-67 staining, after use of the liquid-based cytology samples for a previous cytology slide and several other molecular tests. Alternatively, significant lesions may have yielded hypocellular slides due to excess blood and debris clogging the filter pores. A third explanation is the fact that, for logistic reasons a proportion of liquid-based cytology samples was obtained directly before colposcopy. In these cases, cautious scraping by the physician (to avoid cervical bleeding during colposcopy) might have led to insufficient cell numbers.34 This was illustrated by the fact that the rate of non-evaluable specimens for p16/Ki-67 dual-stained cytology was 22% (56/252) among scrapes, which were obtained directly before colposcopy, compared with 11% (32/283) among scrapes that were collected in a separate visit 2–3 weeks before colposcopy.
A previous study has shown that the interpretation of p16/Ki-67 dual-stained slides is reproducible as well, if performed by non-expert staff in cervical cytology,15 implying that the use of p16/Ki-67 dual-stained cytology could improve the standardization of cervical screening in settings with a lack of cytological expertise. This more objective nature might also be of value, in particular in the triage of high-risk HPV-positive women, in which prior knowledge of high-risk HPV presence could result in a scoring ‘bias’ that likely will decrease specificity of Pap cytology,32, 33 as was also observed in the present study. Yet, it should be realized that p16/Ki-67 dual-stained cytology remains microscopy-based, in contrast to other suggested molecular triage tests.
To support the clinical implementation of p16/Ki-67 dual-stained cytology, more data on the long-term ≥CIN3 risk after a negative p16/Ki-67 dual-stained cytology result are required. The currently available data are promising; in the present cross-sectional study, p16/Ki-67 dual-stained cytology tended to have a low complemented negative predictive value for ≥CIN3 compared with Pap cytology (2.6 vs 5.7%), whereas in a 2-year follow-up schedule, Wentzensen et al31 found an even lower residual ≥CIN3 risk among p16/Ki-67 dual-stain-negative women (complemented negative predictive value 0.6% and 95% CI: 0.2–2.0%).
In summary, p16/Ki-67 dual-stained cytology may serve as a more objective alternative to Pap cytology for triage of high-risk HPV-positive women.
References
Steenbergen RD, Snijders PJ, Heideman DA et al. Clinical implications of (epi)genetic changes in HPV-induced cervical precancerous lesions. Nat Rev Cancer 2014;14:395–405.
Walboomers JM, Jacobs MV, Manos MM et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol 1999;189:12–19.
Ronco G, Dillner J, Elfström KM et al. Efficacy of HPV-based screening for prevention of invasive cervical cancer: follow-up of four European randomised controlled trials. Lancet 2014;383:524–532.
Dijkstra MG, Van Niekerk D, Rijkaart DC et al. Primary hrHPV DNA testing in cervical cancer screening: How to manage screen-positive women? a POBASCAM trial substudy. Cancer Epidemiol Biomarkers Prev 2014;23:55–63.
Rijkaart DC, Berkhof J, van Kemenade FJ et al. Evaluation of 14 triage strategies for HPV DNA-positive women in population-based cervical screening. Int J Cancer 2012;130:602–610.
Massad LS, Einstein MH, Huh WK et al. 2012 Updated consensus guidelines for the management of abnormal cervical cancer screening tests and cancer precursors. J Low Genit Tract Dis 2013;17:S1–27.
Castle PE, Stoler MH, Wright TC et al. Performance of carcinogenic human papillomavirus (HPV) testing and HPV16 or HPV18 genotyping for cervical cancer screening of women aged 25 years and older: a subanalysis of the ATHENA study. Lancet Oncol 2011;12:880–890.
de Sanjose S, Quint WG, Alemany L et al. Human papillomavirus genotype attribution in invasive cervical cancer: a retrospective cross-sectional worldwide study. Lancet Oncol 2010;11:1048–1056.
von Knebel Doeberitz M . New markers for cervical dysplasia to visualise the genomic chaos created by aberrant oncogenic papillomavirus infections. Eur J Cancer 2002;38:2229–2242.
Cuschieri K, Wentzensen N . Human papillomavirus mRNA and p16 detection as biomarkers for the improved diagnosis of cervical neoplasia. Cancer Epidemiol Biomarkers Prev 2008;17:2536–2545.
Petry KU, Schmidt D, Scherbring S et al. Triaging Pap cytology negative, HPV positive cervical cancer screening results with p16/Ki-67 dual-stained cytology. Gynecol Oncol 2011;121:505–509.
Carozzi F, Gillio-Tos A, Confortini M et al. Risk of high-grade cervical intraepithelial neoplasia during follow-up in HPV-positive women according to baseline p16-INK4A results: a prospective analysis of a nested substudy of the NTCC randomised controlled trial. Lancet Oncol 2013;14:168–176.
Wentzensen N, Schwartz L, Zuna RE et al. Performance of p16/Ki-67 immunostaining to detect cervical cancer precursors in a colposcopy referral population. Clin Cancer Res 2012;18:4154–4162.
Schmidt D, Bergeron C, Denton KJ et al. p16/ki-67 dual-stain cytology in the triage of ASCUS and LSIL papanicolaou cytology: results from the European equivocal or mildly abnormal Papanicolaou cytology study. Cancer Cytopathol 2011;119:158–166.
Allia E, Ronco G, Coccia A et al. Interpretation of p16(INK4a) /Ki-67 dual immunostaining for the triage of human papillomavirus-positive women by experts and nonexperts in cervical cytology. Cancer Cytopathol 2015;123:212–218.
Luttmer R, De Strooper LM, Berkhof J et al. Comparing the performance of FAM19A4 methylation analysis, cytology and HPV16/18 genotyping for the detection of cervical (pre)cancer in high-risk HPV-positive women of a gynecologic outpatient population (COMETH study). Int J Cancer 2016;138:992–1002.
Hesselink AT, Heideman DA, Steenbergen RD et al. Combined promoter methylation analysis of CADM1 and MAL: An objective triage tool for high-risk human papillomavirus DNA-positive women. Clin Cancer Res 2011;17:2459–2465.
Jacobs MV, Snijders PJ, Van den Brule AJ et al. A general primer GP5+/GP6+-mediated PCR-enzyme immunoassay method for rapid detection of 14 high-risk and 6 low-risk human papillomavirus genotypes in cervical scrapings. J Clin Microbiol 1997;35:791–795.
Schmitt M, Bravo IG, Snijders PJ et al. Bead-based multiplex genotyping of human papillomaviruses. J Clin Microbiol 2006;44:504–512.
Luttmer R, Berkhof J, Dijkstra MG et al. Comparing triage algorithms using HPV DNA genotyping, HPV E7 mRNA detection and cytology in high-risk HPV DNA-positive women. J Clin Virol 2015;67:59–66.
Hanselaar AG . Criteria for organized cervical screening programs. Special emphasis on The Netherlands program. Acta Cytol 2002;46:619–629.
Uijterwaal MH, Polman NJ, Witte BI et al. Triaging HPV-positive women with normal cytology by p16/Ki-67 dual-stained cytology testing: baseline and longitudinal data. Int J Cancer 2015;136:2361–2368.
Uijterwaal MH, Witte BI, Van Kemenade FJ et al. Triaging borderline/mild dyskaryotic Pap cytology with p16/Ki-67 dual-stained cytology testing: cross-sectional and longitudinal outcome study. Br J Cancer 2014;110:1579–1586.
Tang NS, Tang ML, Chan IS . On tests of equivalence via non-unity relative risk for matched-pair design. Stat Med 2003;22:1217–1233.
Meijer CJ, Berkhof J, Castle PE et al. Guidelines for human papillomavirus DNA test requirements for primary cervical cancer screening in women 30 years and older. Int J Cancer 2009;124:516–520.
Trimble CL, Piantadosi S, Gravitt P et al. Spontaneous regression of high-grade cervical dysplasia: effects of human papillomavirus type and HLA phenotype. Clin Cancer Res 2005;11:4717–4723.
Ostör AG . Natural history of cervical intraepithelial neoplasia: a critical review. Int J Gynecol Pathol 1993;12:186–192.
Ikenberg H, Bergeron C, Schmidt D et al. Screening for cervical cancer precursors with p16/Ki-67 dual-stained cytology: Results of the PALMS study. J Natl Cancer Inst 2013;105:1550–1557.
Kisser A, Zechmeister-Koss I . A systematic review of p16/Ki-67 immuno-testing for triage of low grade cervical cytology. Br J Obstet Gynaecol 2015;122:64–70.
Ordi J, Sagasta A, Munmany M et al. Usefulness of p16/Ki67 immunostaining in the triage of women referred to colposcopy. Cancer Cytopathol 2014;122:227–235.
Wentzensen N, Fetterman B, Castle PE et al. p16/Ki-67 dual stain cytology for detection of cervical precancer in HPV-positive women. J Natl Cancer Inst 2015;107:djv257.
Moriarty AT, Nayar R, Arnold T et al. The Tahoe Study: bias in the interpretation of papanicolaou test results when human papillomavirus status is known. Arch Pathol Lab Med 2014;138:1182–1185.
Bergeron C, Giorgi-Rossi P, Cas F et al. Informed cytology for triaging HPV-positive women: substudy nested in the NTCC randomized controlled trial. J Natl Cancer Inst 2015;107:dju423.
Overmeer RM, Louwers JA, Meijer CJ et al. Combined CADM1 and MAL promoter methylation analysis to detect (pre-)malignant cervical lesions in high-risk HPV-positive women. Int J Cancer 2011;129:2218–2225.
Acknowledgements
We are grateful to the women who participated in this study. We thank the technicians and research staff of the unit of Molecular Pathology of the Department of Pathology at the VU University Medical Center, especially Marjolein Bekker-Lettink, Martijn Bogaarts, and Helma de Bruin. We also thank the cytotechnicians of the Department of Pathology at the VU University Medical Center for cytological testing and logistics, and the administrative workers and information technology team of the Department of Pathology at the VU University Medical Center for the supportive work. We thank the gynecologists, nurses, and administrative workers of the Gynecology Departments of the VU University Medical Center, UMC Utrecht Cancer Center, Onze Lieve Vrouwe Gasthuis, Reinier de Graaf Group, Sint Antonius Hospital, and Flevo Hospital, for their contribution. This study was supported by grants from the Dutch Cancer Society (KWF VU2006-3570, KWF VU2014-7238, and KWF2009-4413) and the Seventh Framework Program of DG Research of the European Commission (CoheaHr-Health-F3-2013-603019). Roche mtm Laboratories provided p16/Ki-67 staining and evaluation of p16/Ki-67 results free of charge.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
PJFS has been on the speakers bureau of Roche, Qiagen, Abbott, Gen-Probe, and Seegene. PJFS is consultant for Crucell Holland B.V. JB has played an advisory role for Merck and Roche, has been on the speakers bureau of Qiagen, and has received a travel reimbursement from DDL Diagnostic Laboratory. TJMH, RV, and WAH have been principle investigators of a GlaxoSmithKline sponsored study. WGVQ is a minority shareholder of Diassay B.V. and obtained grants from GlaxoSmithKline. DAMH has been on the speakers bureau of Hologic/Gen-Probe and serves on the scientific advisory boards of AMGEN and Pfizer. CJLMM has been on the sponsored speakers bureau of GlaxoSmithKline, Qiagen, Merck, Roche, Menarini, and Segeene, and has served on the scientific advisory board of GlaxoSmithKline, Qiagen, Merck, and Roche. CJLMM has been consultant for Qiagen and Genticel, and is a minority shareholder of Diassay B.V. Formerly, CJLMM was a minority shareholder of Delphi Biosciences. CJLMM, PJFS, and DAMH have minority stake in Self-screen B.V., a spin-off company of VU University Medical Center Amsterdam. RL, MGD, FJK, LR, WMB, GCMG, JWMS, and DKED declare no conflicts of interest.
Rights and permissions
About this article
Cite this article
Luttmer, R., Dijkstra, M., Snijders, P. et al. p16/Ki-67 dual-stained cytology for detecting cervical (pre)cancer in a HPV-positive gynecologic outpatient population. Mod Pathol 29, 870–878 (2016). https://doi.org/10.1038/modpathol.2016.80
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/modpathol.2016.80
This article is cited by
-
Combining HPV DNA load with p16/Ki-67 staining to detect cervical precancerous lesions and predict the progression of CIN1–2 lesions
Virology Journal (2019)
-
Dual staining for p16/Ki67 is a more specific test than cytology for triage of HPV-positive women
Virchows Archiv (2018)
-
Evaluation of p16/Ki-67 dual-stained cytology as triage test for high-risk human papillomavirus-positive women
Modern Pathology (2017)
-
Moderne Biomarker bei Präkanzerosen der Cervix uteri
Der Pathologe (2016)