Abstract
Somatostatin receptor 2A expression is a feature of well-differentiated neuroendocrine neoplasms and is important for their diagnosis and therapy. Little is known about somatostatin receptor 2A expression in poorly differentiated neuroendocrine neoplasms in relation to TP53 and RB1 status and how these features may contribute to the separation of well from poorly differentiated neuroendocrine neoplasms with a proliferation index above 20%. This study investigates the expression of somatostatin receptors, p53 and Rb1, and TP53 alterations in pancreatic and extrapancreatic well and poorly differentiated neuroendocrine neoplasms (Ki67-index >20%). Thirty-seven poorly differentiated neuroendocrine neoplasms of pancreatic (n=12) and extrapancreatic origin (n=25) as well as 10 well-differentiated neuroendocrine neoplasms of the pancreas (n=9) and rectum (n=1) with a Ki67-index >20% were immunostained for synaptophysin, chromogranin A, Ki67, CD56, p53, Rb1, ATRX, DAXX, progesterone receptor, somatostatin receptor 2A, somatostatin receptor 5, and cytokeratin 20, and sequenced for TP53, exons 5–9. Somatostatin receptor 2A was positive in 6/37 of poorly differentiated and in 8/10 of well-differentiated neuroendocrine neoplasms. One well-differentiated and two poorly differentiated neuroendocrine neoplasms expressed somatostatin receptor 5. Abnormal nuclear p53 and Rb1 staining was found in 29/37 and 22/37 poorly differentiated neuroendocrine neoplasms, respectively, whereas all well-differentiated neuroendocrine neoplasms showed normal p53 and Rb1 expression. TP53 gene alterations were restricted to poorly differentiated neuroendocrine neoplasms (24/34) and correlated well with p53 expression. All cases were progesterone receptor negative. Somatostatin receptor 2A expression is not limited to well-differentiated neuroendocrine neoplasms but also occurs in 16% of poorly differentiated neuroendocrine neoplasms from various sites. Most poorly differentiated neuroendocrine neoplasms are characterized by TP53 alterations and Rb1 loss, usually in the absence of somatostatin receptor 2A expression. In the pancreas, these criteria contribute to separate well-differentiated neuroendocrine neoplasms with a Ki67-index above 20% from poorly differentiated neuroendocrine neoplasms.
Similar content being viewed by others
Main
The 2010 World Health Organization tumor classification of the gastroenteropancreatic system for neuroendocrine neoplasms defines the tumors with well-differentiated histology and a Ki67-index below 20% as neuroendocrine tumors. In contrast, poorly differentiated neuroendocrine neoplasms that are called neuroendocrine carcinomas have a Ki67-index greater than 20%. Arguments in favor of this distinction are profound differences in tumor progression (slow vs fast), hormone production (expression vs no expression), hormonal syndromes (syndromes vs no syndromes), association with hereditary endocrine tumor syndromes (such as multiple endocrine neoplasia type 1, von Hippel Lindau or neurofibromatosis type 1 vs no association), and genetic profiles (MEN1, DAXX/ARTX, TSC2, PTEN, PIK3CA and VHL gene alterations vs TP53 and RB1 gene alterations).1, 2, 3, 4, 5 This subdivision of the neuroendocrine neoplasms into well and poorly differentiated groups probably also applies to the respective neoplasms outside the gastroenteropancreatic organ system as for instance in the lung (typical and atypical carcinoids vs poorly differentiated neuroendocrine carcinomas), head and neck area or the urinary bladder.
The subdivision of the neuroendocrine neoplasms into two biologically distinct groups is crucial for the treatment of these tumors.6, 7 Neuroendocrine tumors are initially treated by surgery that is usually followed by various bioresponse modifiers such as somatostatin analogs, chemotherapy with streptozotocin, temozolomid and/or peptide radio receptor therapy, if the tumors pursue a malignant course.1 Poorly differentiated neuroendocrine neoplasms are rarely resected, but treated with platinum-based chemotherapy.6 In recent years, peptide radio receptor therapy has gained increasing importance as an effective treatment option for neuroendocrine tumors, provided that the tumors express somatostatin receptors, as the ligand consists of a somatostatin analog coupled to a radioisotope such as yttrium or lutetium.8, 9 Strong membranous immunohistochemical expression highly correlates with somatostatin receptor imaging.10, 11, 12, 13 Somatostatin analogs are well established in the treatment of neuroendocrine tumors due to their inhibition of hormonal symptoms and their anti-proliferative effect.14 The progression free survival rate in a subset of metastatic G1 and G2 enteropancreatic neuroendocrine tumors is longer when treated with somatostatin analogs.15
The majority of gastroenteropancreatic neuroendocrine tumors express somatostatin receptor subtypes 1–5 that can be detected by real time polymerase chain reaction and/or immunohistochemistry.16, 17, 18 Especially somatostatin receptor 2 A is highly expressed in these tumors, whereas the other somatostatin receptors, for instance somatostatin receptor 5, have been less often demonstrated.16, 19 Somatostatin receptor 2 A has also been found to be expressed in neuroendocrine tumors/carcinoids and neuroendocrine carcinomas outside the gastroenteropancreatic system, such as in the lung and in the urinary bladder.19, 20, 21, 22, 23 In contrast to neuroendocrine tumors, the poorly differentiated gastroenteropancreatic neuroendocrine neoplasms do rarely express somatostatin receptor 2 A, thereby usually excluding these neoplasms from the benefits of peptide radio receptor therapy. However, as exceptions from this general notion occur, it is of interest to get a more precise overview of the ability of poorly differentiated neuroendocrine neoplasms of the gastroenteropancreatic system and beyond to express somatostatin receptor 2 A or somatostatin receptor 5, in particular regarding the predictability of the expression related to criteria such as morphology, proliferative activity, and tumor localization.
Recently, the distinction of well from poorly differentiated neuroendocrine neoplasms by applying the World Health Organization 2010 classification3 has become difficult for those few well-differentiated neuroendocrine neoplasms whose Ki67-index exceeds 20%. Although they have retained their well-differentiated neuroendocrine growth pattern, their Ki67-index greater than 20% gives them a grade G3 that shifts them into the poorly differentiated neuroendocrine neoplasm category.24 Neoplasms of this type most often originate in the pancreas and increase their proliferative activity when developing liver metastases in the course of the disease. As their behavior is still better than that of the poorly differentiated neuroendocrine neoplasms, and also their treatment response to first-line platinum-based chemotherapy, as used for poorly differentiated neuroendocrine neoplasms, is often poor, there is a need to distinguish these ‘neuroendocrine tumors G3’ clearly from poorly differentiated neuroendocrine neoplasms.25, 26 In case the histological pattern is ambiguous, surrogate markers could facilitate the differential diagnosis. Recent studies have shown that poorly differentiated neuroendocrine neoplasms of the pancreas often overexpress p53, show TP53 mutations and loose the expression of Rb1, whereas pancreatic neuroendocrine tumors lack these changes but show instead mutations in the MEN1 gene, DAXX/ATRX genes, genes belonging to the mTOR pathway and von Hippel Lindau related genes.2, 27, 28
However, little is known whether neuroendocrine tumors G3 retain the features of the neuroendocrine tumors G1 and G2 with intact TP53 and RB1 function or if these tumors assume an intermediate status between neuroendocrine tumors and poorly differentiated neuroendocrine neoplasms. In addition, it is not clear so far whether they also retain the ability to express somatostatin receptor 2 A and thus may respond to peptide radio receptor therapy.
In this study, we therefore addressed three questions. Firstly, do poorly differentiated neuroendocrine neoplasms from various sites including the pancreas, the colorectum, the urinary bladder, the parotid gland, and the skin (ie, Merkel cell carcinomas) lack the immunohistochemical expression of somatostatin receptor 2 A and 5? Secondly, how does the TP53 and RB1 status relate to the expression of somatostatin receptor 2 A and 5 in poorly differentiated neuroendocrine neoplasms? Thirdly, how helpful are the TP53, RB1, ATRX, and DAXX mutational status and somatostatin receptor 2 A/5 expression patterns in discriminating neuroendocrine tumors G3 from poorly differentiated neuroendocrine neoplasms in the pancreas? The answers to these questions reveal somatostatin receptor 2 A expression in association with intact TP53 and RB1 status as diagnostically valuable neuroendocrine tumor G3 features. P53 and Rb1 have a high discriminative power and are helpful markers to distinguish well from poorly differentiated neuroendocrine neoplasms.
Materials and methods
Patients and Tissues
Formalin-fixed and paraffin-embedded tissue blocks from 47 surgical resection specimens of primary neuroendocrine neoplasms with a Ki67-index >20% were retrieved from the Institute of Pathology and the Consultation Center of Pancreatic and Endocrine Tumors, Institute of Pathology, Technical University Munich, Munich, Germany, including 21 neoplasms of pancreatic (n=20) and ampullary origin (n=1) and 26 neoplasms of extrapancreatic origin (colorectum, n=10; urinary bladder, n=8; parotid gland, n=5; Merkel cell carcinoma of the skin, n=3). The study was approved by the ethics committee of the Technical University of Munich, Germany (document no. 129/16 S).
Histological Analysis
Three micrometer-thick slides were cut from each block and stained with hematoxylin and eosin. The tumors were histologically classified according to World Health Organization recommendations as well-differentiated neuroendocrine neoplasms, when presenting with typical neuroendocrine growth pattern (diffuse, nested, trabecular, glandular, and so on) and low-grade cytological atypia. Poorly differentiated neoplasms of the pancreas, the colorectum, the urinary bladder, the parotid gland, and the skin were subclassified into neoplasms from small cell, large cell, and Merkel cell type, based on cytological criteria. Small-cell-type neoplasms consist of small neoplastic cells with a high nucleus to cytoplasm ratio and dense chromatin. Large-cell-type neoplasms are defined by larger nuclei with vesicular chromatin and large nucleoli.3, 29
Immunohistochemistry
Immunohistochemistry was performed on 3 μm-thick paraffin sections using a fully automated slide preparation system ‘Benchmark XT System’ (Ventana Medical Systems, Tucson, Arizona, USA). All reagents and buffers were retrieved from Ventana Medical Systems. Immunohistochemical analysis was performed using antibodies against synaptophysin (Ventana Medical Systems, Tucson, Arizona, USA; 1:1), chromogranin A (Boehringer, Mannheim, Germany; 1:5000), Ki67 (DakoCytomation, Glostrup, Denmark; 1:50), CD56 (Cell Marque, Rocklin, California, USA; 1:4), p53 (DakoCytomation, Glostrup, Denmark; 1:200), progesterone receptor (DCS, Hamburg, Germany; 1:50), somatostatin receptor 2 A (ZYTOMED Systems, Berlin, Germany; 1:100), somatostatin receptor 5 (ZYTOMED Systems, Berlin, Germany; 1:25), cytokeratin 20 (PROGEN Biotechnik GmbH, Heidelberg, Germany; 1:60), and Rb1 (BD Biosciences, Heidelberg, Germany; 1:200). In addition, ATRX (Sigma-Aldrich, Munich, Germany, 1:250) and DAXX (Sigma-Aldrich, Munich, Germany, 1:100) immunostainings were performed in pancreatic neuroendocrine neoplasms. Nuclear (Ki67, p53, progesterone receptor, Rb1, ATRX, and DAXX), cytoplasmic (synaptophysin, chromogranin A, cytokeratin 20), and membranous (CD56, somatostatin receptor 2 A, somatostatin receptor 5) staining were scored as specific. The grading of the neoplasms was based on the Ki67-index. In poorly differentiated neuroendocrine neoplasms with a Ki67-index >50% a minimum of 250–500 neoplastic cells were counted in hot-spot areas by two observers (GK and BK). In cases of well-differentiated neuroendocrine neoplasms and poorly differentiated neuroendocrine neoplasms with Ki67-index <50%, Ki67-positive cells were manually counted on prints of photographs containing >500 cells and including hot-spot areas.30 P53 expression was scored as abnormal in cases of moderate to strong nuclear positivity in more than 20% of neoplastic cells or in cases of complete absence. The loss of nuclear Rb1, ATRX, and DAXX expression in >90% of neoplastic cells was considered as abnormal. The staining of somatostatin receptors was evaluated using a three tired scoring system (using the established HER2 scoring system of the breast, adapted to the somatostatin receptor 2 A expression patterns in the pancreas). Score 0 (no staining, weakly membranous staining in <10% of neoplastic cells) and score 1+ (weakly membranous staining in >10% of neoplastic cells) were recorded as negative, score 2+ (moderate membranous staining in >10% of neoplastic cells), and score 3+ (strong membranous staining in >10% of neoplastic cells) were recorded as positive.31, 32
Molecular Sequencing
Neoplastic tissue was microdissected from representative formalin-fixed and paraffin-embedded tissue blocks. Genomic DNA was extracted using the FFPE Tissue Kit (Qiagen). Exon 5–9 of the TP53 gene locus were amplified by polymerase chain reaction using the following primers: 5′-atc tgt tca ctt gtg ccc tg-3′ (exon 5 forward), 5′-aac cag ccc tgt cgt ctc tc-3′ (exon 5 reverse), 5′-agg gtc ccc agg cct ctg at-3′ (exon 6 forward), 5′-cac cct taa ccc ctc ctc cc-3′ (exon 6 reverse), 5′-cca agg cgc act ggc ctc atc-3′ (exon 7 forward), 5′-cag agg ctg ggg cac agc agg-3′ (exon 7 reverse), 5′-ttc ctt act gcc tct tgc tt-3′ (exon 8 forward), 5′-tgt cct gct tgc tta cct cg-3′ (exon 8 reverse), 5′-aag caa gca gga caa gaa gc-3′ (exon 9 forward), 5′-cca ctt gat aag agg tcc ca-3′ (exon 9 reverse). The polymerase chain reaction products were directly Sanger sequenced (forward and reverse). Selected cases were additionally analyzed by massive parallel sequencing using a panel of 409 genes including all coding regions of TP53, RB1, KRAS, CDKN2A/p16, SMAD4, RET, MEN1, ATRX, DAXX, and BRAF amongst others on an Ion Torrent S5XL platform.
Statistical Analysis
Statistical analysis was done using SPSS 23.0 statistical software (SPSS, Chicago, IL, USA). Histological analysis was correlated with immunohistochemical findings and TP53 molecular pathology using the Chi-square and Fisher’s exact test. Significance was considered if the P-value was <0.05.
Results
A total of 47 primary neuroendocrine neoplasms with Ki67-index exceeding 20% were examined. The mean age of the patients at diagnosis was 62 years (range 16–89 years). 57% of the patients were women and 43% were men (27:20).
Histological Features
Twelve of 21 (57%) pancreatic neuroendocrine neoplasms (including one ampullary neuroendocrine neoplasm) and 25/26 (96%) extrapancreatic neuroendocrine neoplasms (9 from the colorectum, 8 from the urinary bladder, 5 from the parotid gland, 3 from the skin (ie, Merkel cell carcinomas)) were classified as poorly differentiated neuroendocrine neoplasms. Small-cell-type poorly differentiated neuroendocrine neoplasms were found in the pancreas (4/12) and particularly in the urinary bladder (7/8). Large-cell-type poorly differentiated neuroendocrine neoplasms were seen in the pancreas (8/12) and in the colorectum (7/9). The pancreatic large cell neuroendocrine neoplasm with the lowest proliferation (Ki67-index 21%; case 5 on Table 2) showed a diffuse non-organoid growth pattern (see also Discussion). All neuroendocrine neoplasms of the skin and the parotid gland were classified as Merkel cell- and/or Merkel cell-like carcinomas.29 Three poorly differentiated neuroendocrine neoplasms from the pancreas (n=2) and the rectum (n=1) had a glandular component, two poorly differentiated neuroendocrine neoplasms (one from the parotid gland, one from the urinary bladder) had a squamoid cell component and one poorly differentiated neuroendocrine neoplasm (urinary bladder) had a transitional cell component, exceeding 30% of the tumor cells, and were therefore classified as mixed carcinomas.
Ten of the 47 neuroendocrine neoplasms (nine from the pancreas, one from the rectum) presented with well-differentiated neuroendocrine growth patterns, mainly with a trabecular arrangement, and were classified as well-differentiated neuroendocrine neoplasms or neuroendocrine tumors G3. There was no neuroendocrine tumor G3 that in addition to the well-differentiated component included poorly differentiated areas.
Immunohistochemical Data in Poorly Differentiated Neuroendocrine Neoplasms
All neoplasms were synaptophysin positive. Chromogranin A was expressed in 24 of 37 cases. CD56 was expressed in 9/12 pancreatic and in 24/25 of extrapancreatic poorly differentiated neuroendocrine neoplasms with a focal/weak (10/33) or diffuse/strong (23/33) expression pattern. Cytokeratin 20 was detected in 27/37 neoplasms. The Ki67-index ranged from 21% to 90%.
Around 16% (6/37, pancreas, colon, rectum, urinary bladder, parotid gland) of poorly differentiated neuroendocrine neoplasms were somatostatin receptor 2 A positive (score 2+, 5/6; score 3+ 1/6). And, 32% of the somatostatin receptor 2 A negative cases (10/31) showed a weak membranous staining in more than 10% of neoplastic cells, corresponding to a score of 1+. Somatostatin receptor 5 was expressed in two cases (score 2+, pancreas, colon). Abnormal p53 immunolabeling was seen in 75% (9/12) of pancreatic and in 80% (20/25) of extrapancreatic cases. Loss of nuclear Rb1 expression was more frequent in extrapancreatic (68%, 17/25) than in pancreatic neoplasms (42%, 5/12). All cases of the urinary bladder (100%, 8/8) showed an abnormal Rb1 status, followed by the skin (67%, 2/3), the parotid gland (60%, 3/5), and the colorectum (44%, 4/9). All cases were progesterone receptor negative. All poorly differentiated pancreatic neuroendocrine neoplasms showed a normal nuclear ATRX and DAXX staining (both 100%, 11/11).
Immunohistochemical Data in Well-Differentiated Neuroendocrine Neoplasms with a Ki67-Index Greater than 20% (Neuroendocrine Tumors G3)
All neoplasms were synaptophysin and chromogranin A positive. The Ki67-index ranged from 21% to 36%. Eight of ten neuroendocrine tumors G3 were somatostatin receptor 2 A positive (score 2+, 6/8; score 3+, 2/8). Somatostatin receptor 5 (score 2+) was expressed in a single pancreatic neuroendocrine tumor G3. No abnormal immunostaining of p53 and Rb1 was detected. CD56 and cytokeratin 20 were expressed in all neuroendocrine tumors G3. All cases were progesterone receptor negative. Nuclear ATRX and DAXX losses were seen in 1/9 and in 3/9 pancreatic neuroendocrine tumors G3, respectively.
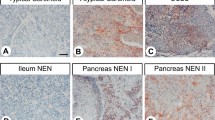
Figures 1 and 2 show the histological and immunohistochemical stainings for somatostatin receptor 2 A, Ki67, and Rb1 of extrapancreatic poorly differentiated neuroendocrine neoplasms and pancreatic neuroendocrine neoplasms with a Ki67-index >20%, respectively. Figure 3 shows the ATRX and DAXX immunohistochemistry in pancreatic neuroendocrine carcinomas and neuroendocrine tumors G3.
Immunohistochemistry of extrapancreatic poorly differentiated neuroendocrine carcinomas: (a–c) large cell neuroendocrine carcinoma of the colon (a) with expression of somatostatin receptor 2 A (b, score 2+), a Ki67-index of 60% (b, inset) and normal nuclear expression of Rb1 (c); (d–f) small cell neuroendocrine carcinoma of the urinary bladder (d) with expression of somatostatin receptor 2 A (e, score 3+), a Ki67-index of 90% (e, inset) and nuclear loss of Rb1 (f); (g–i) Merkel cell type neuroendocrine carcinoma of the parotid gland (g) with a somatostatin receptor 2 A score 1+ (h), a Ki67-index of 90% (h, inset) and nuclear loss of Rb1 (i).
Immunohistochemistry of pancreatic neuroendocrine neoplasms with a Ki67-index above 20%: (a–c) small cell neuroendocrine carcinoma (a) with a somatostatin receptor 2 A score 1+ (b), a Ki67-index of 55% (b, inset) and normal nuclear expression of Rb1 (c); (d–f) large cell neuroendocrine carcinoma (d) with expression of somatostatin receptor 2 A (e, score 2+), a Ki67-index of 70% (e, inset) and nuclear loss of Rb1 (f); (g–i) neuroendocrine tumor G3 (g) with expression of somatostatin receptor 2 A (h, score 2+), a Ki67-index of 21% (h, inset) and normal nuclear expression of Rb1 (i).
Immunohistochemistry of ATRX and DAXX in pancreatic neuroendocrine neoplasms with a Ki67-index above 20%: (a and b) large cell neuroendocrine carcinomas of the pancreas with normal nuclear expression of ATRX (a) and DAXX (b); (c and d) neuroendocrine tumors G3 with nuclear loss of ATRX (c) and DAXX (d), endothelial cells served as positive internal controls.
Molecular TP53 Status
Molecular analysis of exons 5–9 of the TP53 gene was performed in 44/47 cases (three cases of poorly differentiated neuroendocrine neoplasms of the urinary bladder were not available for molecular analysis). 71% (24/34) of poorly differentiated neuroendocrine neoplasms harbored TP53 mutations. Exon 5 was altered in 38% (9/24), exon 6 in 8% (2/24), exon 7 in 25% (6/24), exon 8 in 25% (6/24), and exon 9 in 13% (3/24) of the cases. One parotid gland neuroendocrine carcinoma and one Merkel cell carcinoma showed mutations both in exons 5 and 9, and in exons 6 and 8, respectively. Most mutations were missense mutations (58%, 15/26), followed by nonsense mutations (15%, 4/26), splice site defects (12%, 3/26) and frameshift mutations (12%, 3/26) as well as deletions (4%, 1/26). 64% (7/11) of pancreatic poorly differentiated neuroendocrine neoplasms were TP53 mutated, either in exon 5 (4/7) or exon 7 (3/7). The ampullary poorly differentiated neuroendocrine neoplasm showed a mutation in exon 8. Amongst extrapancreatic poorly differentiated neuroendocrine neoplasms, 73% (16/22) of them were TP53 mutated (2/5 of the parotid gland, 2/3 Merkel cell carcinomas, 8/9 of the colorectum, 4/5 of the urinary bladder). All neuroendocrine tumors G3 were TP53 wild type.
Table 1 summarizes immunohistochemical and molecular findings in poorly differentiated neuroendocrine neoplasms of pancreatic and extrapancreatic origin. Table 2 summarizes the clinicopathological data including immunohistochemistry and molecular analysis of poorly differentiated neuroendocrine neoplasms and neuroendocrine tumors G3 of the pancreas. Three neuroendocrine carcinomas showed a TP53 wild-type status and a normal immunohistochemistry for p53 and Rb1, while their histology was compatible with neuroendocrine carcinoma features (case numbers 1, 3, 5; Table 2). Deep sequencing confirmed the wild-type status of TP53 and RB1, but revealed mutations in case 1, affecting NF1, CSMD3, EP300, KDM5C, AKT3, CDH5, ERBB2, and BIRC5. Case 5, which had a Ki67-index of 21% despite its diffuse non-organoid histology (see also discussion and Figure 5) and a somatostatin receptor 2 A 1+ score, and case 3 had no mutation in any of the genes included in our panel (data not shown).
Correlation Between Somatostatin Receptor 2 A, p53, Rb1, and TP53 Status
Neuroendocrine tumors G3 were significantly more often somatostatin receptor 2 A positive than their poorly differentiated counterparts (P-value: 0.0001) and showed a normal p53 expression (P-value: 0.0001). TP53 wild-type status was significantly associated with well-differentiated morphology (P-value: 0.0001). Somatostatin receptor 2 A positive poorly differentiated neuroendocrine neoplasms harbored TP53 mutations in four of six cases. Abnormal p53 immunolabeling was an indicator for TP53 gene alterations (23/26 cases, 88%, P-value: 0.0001) and often occurred in combination with nuclear Rb1 loss (P-value: 0.001). Loss of nuclear Rb1 was not present in neuroendocrine tumors G3, but was frequently found in neuroendocrine neoplasms with poorly differentiated morphology (P-value: 0.001). Figure 4 shows normal and abnormal nuclear p53 stainings and TP53 mutations.
P53 and TP53 in pancreatic neuroendocrine neoplasms: normal nuclear expression of p53 in <20% of the cells of a neuroendocrine tumor G3 (a); abnormal expression of p53 with complete loss of nuclear staining in a large cell type neuroendocrine carcinoma (b); nuclear overexpression of p53 in a small cell type neuroendocrine carcinoma (c); Sanger sequences of TP53 mutated cases with a frameshift (upper row) and a point mutation (lower row) (d).
Discussion
There is increasing evidence that the low proliferative well-differentiated neuroendocrine neoplasms (G1–2) and the high proliferative poorly differentiated neuroendocrine neoplasms (G3) belong to two biologically different entities of neuroendocrine neoplasms.2 The results of our study confirm and extend this notion. We show that the majority of poorly differentiated neuroendocrine neoplasms, pancreatic as well as extrapancreatic, lack significant somatostatin receptor 2 A expression, a key finding in well-differentiated neuroendocrine neoplasms, and, at the same time, have TP53 and RB1 gene alterations. This dichotomy also applies to those neuroendocrine neoplasms that maintain their well-differentiated histology although their Ki67-index exceeds 20%. Accordingly, these neuroendocrine neoplasms have been provisionally termed ‘neuroendocrine tumors G3’. They are mainly of pancreatic and only rarely of extrapancreatic origin.24, 33, 34
The membranous expression of somatostatin receptor 2 A is a typical feature of well-differentiated neuroendocrine tumors of different origin.18 In contrast, somatostatin receptor 2 A expression in highly proliferative neuroendocrine neoplasms has only been demonstrated in a fraction of tumors from various sites.16, 19, 35 In Kaemmerer’s study investigating somatostatin receptor 2 A expression in gastroenteropancreatic neuroendocrine neoplasms (together with the CXCR4 chemokine receptor), 23% of the tumors regarded to be G3 were strongly somatostatin receptor 2 A positive.16 Using the established HER2 scoring system of the breast and adapting it to the somatostatin receptor 2 A expression patterns in the pancreas,31, 32 our data in poorly differentiated neuroendocrine neoplasms of pancreatic and extrapancreatic origin demonstrated that somatostatin receptor 2 A, scored 2+ and 3+, was positive in 16% of these neoplasms including one in the pancreas and in the parotid gland, as well as two cases each in the colon and the urinary bladder. The positive tumors belonged to the small or large cell category and showed a range between 50% and 90% of proliferative activity. For example, the three pancreatic poorly differentiated neuroendocrine neoplasms with the lowest Ki67-index (between 21% and 35%) were all somatostatin receptor 2 A negative.
Low expression levels of somatostatin receptor 2 A (score 1+) were found in ten poorly differentiated neuroendocrine neoplasms. Miederer showed that few neuroendocrine neoplasms detected by somatostatin receptor imaging were somatostatin receptor 2 A negative.12 This discrepancy between imaging and immunohistochemistry is most likely due to tumor heterogeneity regarding somatostatin receptor 2 A expression which effects imaging, as a large scale method, much less than immunohistochemical examination that screens only a small tumor area. It is therefore possible that among our score 1+ somatostatin receptor 2 A positive poorly differentiated neuroendocrine neoplasms are some tumors that might be detected by imaging.
Somatostatin receptor 5, which shares the membranous expression with somatostatin receptor 2 A, was only found to be expressed in three cases, including two poorly differentiated neuroendocrine neoplasms and one neuroendocrine tumor G3. Somatostatin receptor 5 expression was neither correlated to somatostatin receptor 2 A positivity nor to any of the above mentioned tumor criteria. These data suggest that somatostatin receptor 5 probably does not play any significant functional role in neuroendocrine neoplasms, neither in those with well nor with poorly differentiated histology.
As to tumor localization, it appears from our data and those in other studies that somatostatin receptor 2 A may be present in poorly differentiated neuroendocrine neoplasms from various sites of the body, including the pancreas, stomach, colorectum, urinary bladder, lung, breast, and prostate.16, 19, 20, 21, 35 Whether Merkel cell carcinomas of the skin may be an exception in this regard, as our data might suggest, remains to be clarified in a larger series of cases.
In order to find out whether there is any correlation between the somatostatin receptor 2 A expression with the TP53 and RB1 status, we investigated p53 and Rb1 expression or loss, and TP53 mutation in our tumor series. In confirming a recent genetic analysis of poorly differentiated small cell and large cell neuroendocrine neoplasms of the pancreas,2 we found TP53 mutated in 71% of poorly differentiated neuroendocrine neoplasms and abnormally expressed in 78% of the cases. Rb1 was lost in 59% of the cases (Table 1). Abnormal p53 immunolabeling (ie, overexpression as well as total loss) matched with TP53 gene alterations in 92% of our cases. Correlation of the TP53 data with that of somatostatin receptor 2 A expression revealed no relationship between the two markers.
Histologically, the differential diagnosis of neuroendocrine tumors G3 and poorly differentiated neuroendocrine neoplasms, particularly of the large cell subtype, may sometimes be difficult. In these cases it would be helpful to have immunohistochemical and/or molecular markers available to identify these tumors. The comparison of the results in a series of nine primary pancreatic neuroendocrine tumors G3 with those in twelve pancreatic poorly differentiated neuroendocrine neoplasms shows that the application of somatostatin receptor 2 A, p53, Rb1, ATRX, and DAXX together with a high Ki67-index can solve the problem in most cases. Exceptionally, as observed in case 5 (Table 2; Figure 5), classification as poorly differentiated neuroendocrine neoplasm may remain difficult, if it is only based on histology (showing a diffuse non-organoid growth pattern) and low level somatostatin receptor 2 A expression, and not on TP53 and RB1 alterations or a high Ki67-index. Progesterone receptor expression, which was also tested, was of no use, as it was negative in all cases. The discriminative power in separating neuroendocrine tumors G3 from poorly differentiated neuroendocrine neoplasms was greatest for p53, which, in case of abnormal expression, was associated with poorly differentiated neuroendocrine neoplasms in almost 78% of the neoplasms, and, in case of negativity, with all neuroendocrine tumors G3. A similar but less sensitive distinction was also seen for Rb1. Somatostatin receptor 2 A, with its 80% positivity in neuroendocrine tumors G3, was less discriminative in poorly differentiated neuroendocrine neoplasms than p53 and Rb1, as it was found to be positive in six of the latter neoplasms. However, when somatostatin receptor 2 A was combined with p53 and Rb1, it usually solved the differential diagnosis between pancreatic neuroendocrine tumors G3 and poorly differentiated neuroendocrine neoplasms in most of the cases.
In summary, we showed that the expression of somatostatin receptor 2 A is not restricted to the group of well-differentiated neuroendocrine neoplasms but may also be detected in a small fraction of poorly differentiated neuroendocrine neoplasms of pancreatic and extrapancreatic origin. This makes these neuroendocrine neoplasms potentially amenable to treatment with somatostatin analogs and peptide radio receptor therapy. Morphological criteria that predict somatostatin receptor 2 A positivity in poorly differentiated neuroendocrine neoplasms could not be identified so far. The distinction of neuroendocrine tumors from poorly differentiated neuroendocrine neoplasms, that may be especially difficult in the neuroendocrine tumors G3 of pancreatic origin, can be very much improved by the application of p53 in combination with Rb1 and somatostatin receptor 2 A. ATRX and DAXX are less important as their discriminative power is much lower than that of aforementioned markers. The fact that p53, Rb1 and somatostatin receptor 2 A have such a high differential expression in well and poorly differentiated neuroendocrine neoplasms further supports the concept of two basically different groups among the neuroendocrine neoplasms.5, 34
References
Shi C, Klimstra DS . Pancreatic neuroendocrine tumors: pathologic and molecular characteristics. Semin Diagn Pathol 2014; 31: 498–511.
Yachida S, Vakiani E, White CM et al, Small cell and large cell neuroendocrine carcinomas of the pancreas are genetically similar and distinct from well-differentiated pancreatic neuroendocrine tumors. Am J Surg Pathol 2012; 36: 173–184.
Rindi G, Arnold R, Bosman FT et al, Nomenclature and classification of neuroendocrine neoplasms of the digestive system In: Bosman FT, Carneiro F, Hruban RH, Theise ND (eds). WHO Classification of Tumours of the Digestive System, 4th edn. WHO Press: IARC Lyon, 2010, pp 13–14.
Esposito I, Segler A, Steiger K, et al. Pathology, genetics and precursors of human and experimental pancreatic neoplasms: an update. Pancreatology 2015; 15: 598–610.
Klöppel G . Classification and pathology of gastroenteropancreatic neuroendocrine neoplasms. Endocr Relat Cancer 2011; 18: S1–16.
Sorbye H, Welin S, Langer SW et al, Predictive and prognostic factors for treatment and survival in 305 patients with advanced gastrointestinal neuroendocrine carcinoma (WHO G3): the NORDIC NEC study. Ann Oncol 2013; 24: 152–160.
Frilling A, Akerstrom G, Falconi M et al, Neuroendocrine tumor disease: an evolving landscape. Endocr Relat Cancer 2012; 19: R163–R185.
van Essen M, Krenning EP, Kam BL et al, Peptide-receptor radionuclide therapy for endocrine tumors. Nat Rev Endocrinol 2009; 5: 382–393.
Kwekkeboom DJ, de Herder WW, Kam BL et al, Treatment with the radiolabeled somatostatin analog [177 Lu-DOTA 0,Tyr3]octreotate: toxicity, efficacy, and survival. J Clin Oncol 2008; 26: 2124–2130.
Volante M, Brizzi MP, Faggiano A et al, Somatostatin receptor type 2 A immunohistochemistry in neuroendocrine tumors: a proposal of scoring system correlated with somatostatin receptor scintigraphy. Mod Pathol 2007; 20: 1172–1182.
Kaemmerer D, Peter L, Lupp A et al, Molecular imaging with (6)(8)Ga-SSTR PET/CT and correlation to immunohistochemistry of somatostatin receptors in neuroendocrine tumours. Eur J Nucl Med Mol Imaging 2011; 38: 1659–1668.
Miederer M, Seidl S, Buck A et al, Correlation of immunohistopathological expression of somatostatin receptor 2 with standardised uptake values in 68Ga-DOTATOC PET/CT. Eur J Nucl Med Mol Imaging 2009; 36: 48–52.
Korner M, Waser B, Schonbrunn A et alSomatostatin receptor subtype 2 A immunohistochemistry using a new monoclonal antibody selects tumors suitable for in vivo somatostatin receptor targeting. Am J Surg Pathol 2012; 36: 242–252.
Rinke A, Muller HH, Schade-Brittinger C et al, Placebo-controlled, double-blind, prospective, randomized study on the effect of octreotide LAR in the control of tumor growth in patients with metastatic neuroendocrine midgut tumors: a report from the PROMID Study Group. J Clin Oncol 2009; 27: 4656–4663.
Caplin ME, Pavel M, Cwikla JB et al, Lanreotide in metastatic enteropancreatic neuroendocrine tumors. N Engl J Med 2014; 371: 224–233.
Kaemmerer D, Trager T, Hoffmeister M et al, Inverse expression of somatostatin and CXCR4 chemokine receptors in gastroenteropancreatic neuroendocrine neoplasms of different malignancy. Oncotarget 2015; 6: 27566–27579.
Nakayama Y, Wada R, Yajima N et alProfiling of somatostatin receptor subtype expression by quantitative PCR and correlation with clinicopathological features in pancreatic endocrine tumors. Pancreas 2010; 39: 1147–1154.
Papotti M, Bongiovanni M, Volante M et al, Expression of somatostatin receptor types 1-5 in 81 cases of gastrointestinal and pancreatic endocrine tumors. A correlative immunohistochemical and reverse-transcriptase polymerase chain reaction analysis. Virchows Arch 2002; 440: 461–475.
Mizutani G, Nakanishi Y, Watanabe N et al, Expression of Somatostatin Receptor (SSTR) Subtypes (SSTR-1, 2 A, 3, 4 and 5) in Neuroendocrine Tumors Using Real-time RT-PCR Method and Immunohistochemistry. Acta Histochem Cytochem 2012; 45: 167–176.
Nese N, Kumbaraci BS, Baydar DE et al, Small cell carcinomas of the bladder highly express somatostatin receptor type 2 A: impact on prognosis and treatment-A multicenter study of Urooncology Society, Turkey. Appl Immunohistochem Mol Morphol 2016; 24: 253–260.
Lapa C, Hanscheid H, Wild V et al, Somatostatin receptor expression in small cell lung cancer as a prognostic marker and a target for peptide receptor radionuclide therapy. Oncotarget 2016; 7: 20033–20040.
Kanakis G, Grimelius L, Spathis A et al, Expression of somatostatin receptors 1-5 and dopamine receptor 2 in lung carcinoids: implications for a therapeutic role. Neuroendocrinology 2015; 101: 211–222.
Righi L, Volante M, Tavaglione V et al, Somatostatin receptor tissue distribution in lung neuroendocrine tumours: a clinicopathologic and immunohistochemical study of 218 'clinically aggressive' cases. Ann Oncol 2010; 21: 548–555.
Basturk O, Yang Z, Tang LH et al, The high-grade (WHO G3) pancreatic neuroendocrine tumor category is morphologically and biologically heterogenous and includes both well differentiated and poorly differentiated neoplasms. Am J Surg Pathol 2015; 39: 683–690.
Sorbye H, Strosberg J, Baudin E et alGastroenteropancreatic high-grade neuroendocrine carcinoma. Cancer 2014; 120: 2814–2823.
Velayoudom-Cephise FL, Duvillard P, Foucan L et al, Are G3 ENETS neuroendocrine neoplasms heterogeneous? Endocr Relat Cancer 2013; 20: 649–657.
Jiao Y, Shi C, Edil BH et al, DAXX/ATRX, MEN1, and mTOR pathway genes are frequently altered in pancreatic neuroendocrine tumors. Science 2011; 331: 1199–1203.
Tang LH, Basturk O, Sue JJ, Klimstra DS . A practical approach to the classification of WHO grade 3 (G3) well-differentiated neuroendocrine tumor (WD-NET) and poorly differentiated neuroendocrine carcinoma (PD-NEC) of the pancreas. Am J Surg Pathol 2016; 40: 1192–1202.
Kohler S, Kerl H. Merkel cell carcinoma. In: LeBoit PE, Burg G, Weedon D, Sarasin A (eds). World Health Organization Classification of Tumours. Skin Tumours 1st edn. WHO Press: IARC Lyon, 2006 pp 272–273.
Reid MD, Bagci P, Ohike N et al, Calculation of the Ki67 index in pancreatic neuroendocrine tumors: a comparative analysis of four counting methodologies. Mod Pathol 2015; 28: 686–694.
Kaemmerer D, Peter L, Lupp A et al, Comparing of IRS and Her2 as immunohistochemical scoring schemes in gastroenteropancreatic neuroendocrine tumors. Int J Clin Exp Pathol 2012; 5: 187–194.
Wolff AC, Hammond ME, Hicks DG et al, Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J Clin Oncol 2013; 31: 3997–4013.
Garcia-Carbonero R, Sorbye H, Baudin E et al, ENETS Consensus Guidelines for high-grade gastroenteropancreatic neuroendocrine tumors and neuroendocrine carcinomas. Neuroendocrinology 2016; 103: 186–194.
Tang LH, Untch BR, Reidy DL et al, Well-differentiated neuroendocrine tumors with a morphologically apparent high-grade component: a pathway distinct from poorly differentiated neuroendocrine carcinomas. Clin Cancer Res 2016; 22: 1011–1017.
Zamora V, Cabanne A, Salanova R et al, Immunohistochemical expression of somatostatin receptors in digestive endocrine tumours. Dig Liver Dis 2010; 42: 220–225.
Acknowledgements
We are grateful to Petra Meyer, Daniela Angermeier and Dennis Thiele for excellent technical assistance. We thank Professor Anne Hoorens, Gent, Belgium, Dr Diana Karimi, Munich, Germany, Dr Gratiana Hermann, Zerifin, Israel and Professor Yersu Kapran, Istanbul, Turkey, who supported this study by contributing cases. This study has been supported by a fund from Novartis Pharma GmbH, Germany. KS is funded by DFG (SFB824, Z2).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Konukiewitz, B., Schlitter, A., Jesinghaus, M. et al. Somatostatin receptor expression related to TP53 and RB1 alterations in pancreatic and extrapancreatic neuroendocrine neoplasms with a Ki67-index above 20%. Mod Pathol 30, 587–598 (2017). https://doi.org/10.1038/modpathol.2016.217
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/modpathol.2016.217
This article is cited by
-
Praktische Anwendung von Immunhistochemie in pankreatischen neuroendokrinen Neoplasien
Die Pathologie (2024)
-
Efficacy and tolerability of somatostatin analogues according to gender in patients with neuroendocrine tumors
Reviews in Endocrine and Metabolic Disorders (2024)
-
Clinical practice guidelines for molecular tumor marker, 2nd edition review part 2
International Journal of Clinical Oncology (2024)
-
Potent molecular-targeted therapies for gastro-entero-pancreatic neuroendocrine carcinoma
Cancer and Metastasis Reviews (2023)
-
Integrating Functional Imaging and Molecular Profiling for Optimal Treatment Selection in Neuroendocrine Neoplasms (NEN)
Current Oncology Reports (2023)